Team:BCCS-Bristol/Wetlab/Beads/Gellan
From 2010.igem.org
| (2 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:BCCS-Bristol/Header}} | {{:Team:BCCS-Bristol/Header}} | ||
| - | |||
<center> • [[:Team:BCCS-Bristol/Wetlab/Beads|Overview]] • [[:Team:BCCS-Bristol/Wetlab/Beads/Gellan |Bead Materials]] • [[:Team:BCCS-Bristol/Wetlab/making_beads|Making Beads]] | <center> • [[:Team:BCCS-Bristol/Wetlab/Beads|Overview]] • [[:Team:BCCS-Bristol/Wetlab/Beads/Gellan |Bead Materials]] • [[:Team:BCCS-Bristol/Wetlab/making_beads|Making Beads]] | ||
• [[:Team:BCCS-Bristol/Wetlab/difference_solution|Beads in Solution]] • [[:Team:BCCS-Bristol/Wetlab/difference_soil|Beads in Soil]] • [[:Team:BCCS-Bristol/Wetlab/Beads/ImageGallery|Image Gallery]] • </center> | • [[:Team:BCCS-Bristol/Wetlab/difference_solution|Beads in Solution]] • [[:Team:BCCS-Bristol/Wetlab/difference_soil|Beads in Soil]] • [[:Team:BCCS-Bristol/Wetlab/Beads/ImageGallery|Image Gallery]] • </center> | ||
| - | |||
=Gellan= | =Gellan= | ||
| - | |||
| - | |||
| - | |||
The beads are made out of a gellan gel. Gellan is an extracellular anionic heteropolysaccharide | The beads are made out of a gellan gel. Gellan is an extracellular anionic heteropolysaccharide | ||
| - | produced by Sphingomonas elodea [1]. It is water soluble and gels ionically. The polysaccharides | + | produced by ''Sphingomonas elodea'' [1]. It is water soluble and gels ionically. The polysaccharides |
strongly cross link in the presence of divalent cations forming a robust gel. It is biodegradable | strongly cross link in the presence of divalent cations forming a robust gel. It is biodegradable | ||
and safe for human and animal consumption. It is used in the food industry, often as a texturing | and safe for human and animal consumption. It is used in the food industry, often as a texturing | ||
agent in confectionery. It has an almost identical refractive index to water, meaning that it will | agent in confectionery. It has an almost identical refractive index to water, meaning that it will | ||
| - | transmit light from the | + | transmit light from the fluorescent proteins effectively. It is slightly autofluorescent, though less |
| - | + | autofluorescent than untransformed E. coli. The diffusion constant for small molecules (e.g. nitrate) | |
| - | is | + | is effectively the same as for water, the diffusion constant for larger molecules (e.g. sugars) is slightly |
lower, though within one order of magnitude for equivalent temperatures [2]. This means that it | lower, though within one order of magnitude for equivalent temperatures [2]. This means that it | ||
should help absorb nitrate from the soil and equalize its concentration in the bead. | should help absorb nitrate from the soil and equalize its concentration in the bead. | ||
| - | The procedure for making the gel beads is adapted from standard procedures in cell | + | The procedure for making the gel beads is adapted from standard procedures in cell encapsulation [3]. The gellan is sourced as a powder and is mixed with deionised water and heated to |
| - | + | 90 ◦ C to form a viscous fluid. It is then cooled and mixed with a highly concentrated slurry of cells | |
| - | 90 ◦ C to form a viscous | + | |
when the temperature reaches 56 ◦ C. This mixture is then pipetted into a container of 0.5 molar | when the temperature reaches 56 ◦ C. This mixture is then pipetted into a container of 0.5 molar | ||
| - | room temperature calcium chloride solution in drops of a few hundred | + | room temperature calcium chloride solution in drops of a few hundred micro-litres. The process of |
| - | gelation is rapid, the outside of the drop | + | gelation is rapid, the outside of the drop solidifies as soon as it hits the calcium chloride solution, |
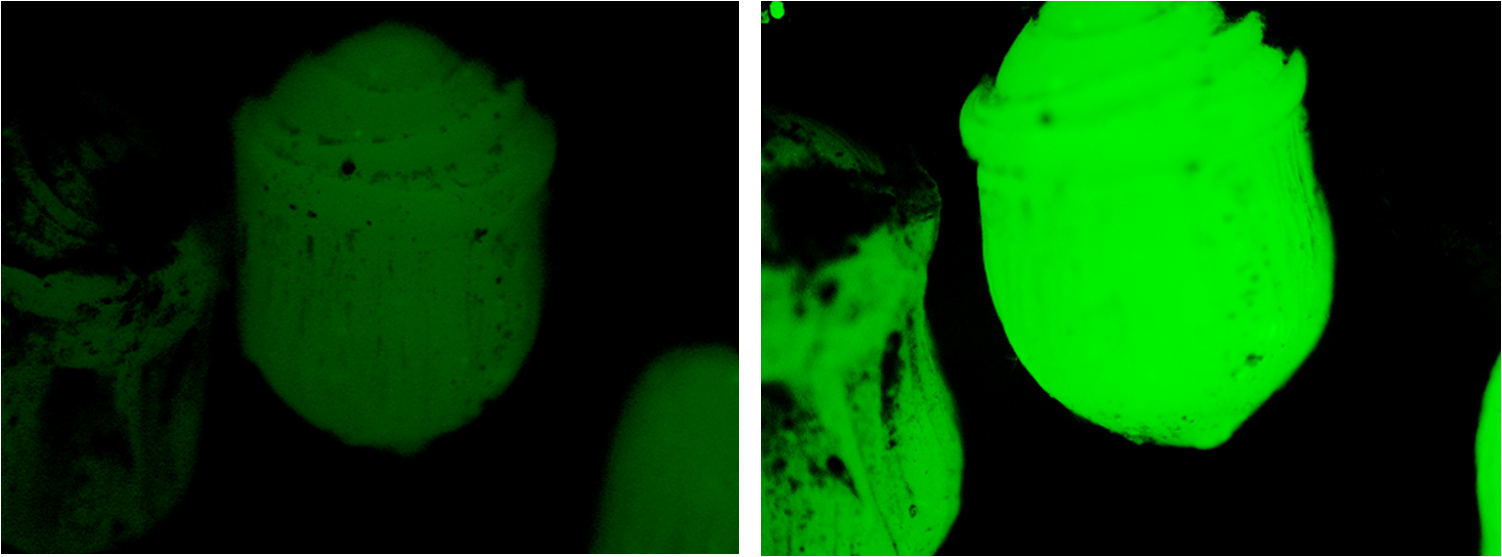
| - | creating a droplet shape with distinctive | + | creating a droplet shape with distinctive 'shockwaves' as can be seen in fig. 1. |
[[image:Bead_diff.png|left|200px|thumb|Fig 1, Glowing beads imaged with a microscope]] | [[image:Bead_diff.png|left|200px|thumb|Fig 1, Glowing beads imaged with a microscope]] | ||
The beads are relatively uniform in size, see fig. 2 for an example of their scale. The beads are stored in nutrient | The beads are relatively uniform in size, see fig. 2 for an example of their scale. The beads are stored in nutrient | ||
| - | broth before use to ensure that the E. coli survive. In [[media:beads.png | this figure]] one can see examples of | + | broth before use to ensure that the ''E. coli'' survive. In [[media:beads.png | this figure]] one can see examples of different beads |
that have been stored, the two tubes on the right for almost 3 weeks, and the two tubes on the left | that have been stored, the two tubes on the right for almost 3 weeks, and the two tubes on the left | ||
for less than 1 week. They appear to stay intact in solution for a period of about ten days. On soil | for less than 1 week. They appear to stay intact in solution for a period of about ten days. On soil | ||
Latest revision as of 19:44, 27 October 2010
iGEM 2010
Gellan
The beads are made out of a gellan gel. Gellan is an extracellular anionic heteropolysaccharide produced by Sphingomonas elodea [1]. It is water soluble and gels ionically. The polysaccharides strongly cross link in the presence of divalent cations forming a robust gel. It is biodegradable and safe for human and animal consumption. It is used in the food industry, often as a texturing agent in confectionery. It has an almost identical refractive index to water, meaning that it will transmit light from the fluorescent proteins effectively. It is slightly autofluorescent, though less autofluorescent than untransformed E. coli. The diffusion constant for small molecules (e.g. nitrate) is effectively the same as for water, the diffusion constant for larger molecules (e.g. sugars) is slightly lower, though within one order of magnitude for equivalent temperatures [2]. This means that it should help absorb nitrate from the soil and equalize its concentration in the bead.
The procedure for making the gel beads is adapted from standard procedures in cell encapsulation [3]. The gellan is sourced as a powder and is mixed with deionised water and heated to
90 ◦ C to form a viscous fluid. It is then cooled and mixed with a highly concentrated slurry of cells
when the temperature reaches 56 ◦ C. This mixture is then pipetted into a container of 0.5 molar
room temperature calcium chloride solution in drops of a few hundred micro-litres. The process of
gelation is rapid, the outside of the drop solidifies as soon as it hits the calcium chloride solution,
creating a droplet shape with distinctive 'shockwaves' as can be seen in fig. 1.
The beads are relatively uniform in size, see fig. 2 for an example of their scale. The beads are stored in nutrient
broth before use to ensure that the E. coli survive. In this figure one can see examples of different beads
that have been stored, the two tubes on the right for almost 3 weeks, and the two tubes on the left
for less than 1 week. They appear to stay intact in solution for a period of about ten days. On soil
they become damaged if they dry out too much, but otherwise retain their shape for a period of
about a week.
[1] et al. S. Patil. Study of formulation variables on properties of drug-gellan beads by factorial
design. Drug Development and Industrial Pharmacy, 32:315-326, 2006.
[2] et al. S. Bayarri. Diffusion of sucrose and aspartame in kappa-carrageenan and gellan gum gels. Food Hydrocolloids, 15:67-73, 2001.
[3] T. M. S. Chang. Procedures for microencapsulation of enzymes, cell and genetically engineered microorganisms. Molecular Biotechnology, 17:249-260, 2001.
 "
"