Team:Monash Australia/Project
From 2010.igem.org

|
|
|||
| Overall project | Ethylene products we can relate to | In simpler terms | Experimental plan | Results |
Overall project
Monash University has a strong push for sustainability and this has rubbed off on us as students. We are passionate about using synthetic biology to reduce the impact humans have on the planet and this inspired our first iGEM project.
A hot topic in Australia is the billions of non-degradable and non-renewable plastic bags that are used every year around the country, which go on to pollute waterways and take up space in landfills already packed to the limit. We decided to come up with a better way to produce poly-ethylene, the most widely used plastic compound. Not only plastic bags, but almost every plastic good imaginable from take-away food containers to rainwater tanks is manufactured from ethylene. Currently, ethylene is produced from petrochemicals and thus comes with a heavy environmental footprint. The way forward, we believe, is to take advantage of the ethylene production systems in plants and thus make ethylene manufacture both renewable and environmentally friendly.
So what is ethylene used for?
Just about everything you do today will have you come in contact with an ethylene based product. One of the most common ethylene based products is the humble plastic shopping bag. They come in all different shapes and sizes and can be found in every country in the world. Ethylene can be polymerised to create polyethylene and is a feedstock for many other plastics, including PVC, polyester and polystyrene. However, it doesn’t stop there: ethylene is also used to produce products as varied as detergent, anti-freeze,, alcohol, cosmetics, and bulletproof vests. Due to its combustible properties, it has considerable promise as a fuel for vehicles. It can even be used to ripen bananas and enhance latex production from rubber trees!
How do we currently make ethylene?
Currently ethylene is produced from oil or natural gas by ‘steam cracking’. This requires a huge amount of energybecause the hydrocarbons must be heated to 750–950 °C, and then immediately quenched to -157 °C to stop free radical reactions. Ethylene is separated from the resulting complex mixture by repeated compression and distillation. The average ethylene production plant has a 34,000 kW cracked gas compressor, a 22,000 kW propylene compressor, and a 11,000 kW ethylene compressor, which combine to one big power bill and an enormous carbon footprint! The fossil fuels used are also non-renewable resources that may soon be in short supply. Oil, particularly, is a major environmental hazard, as we saw recently with the unprecedented spill in the Gulf of Mexico.
How do plants make ethylene?
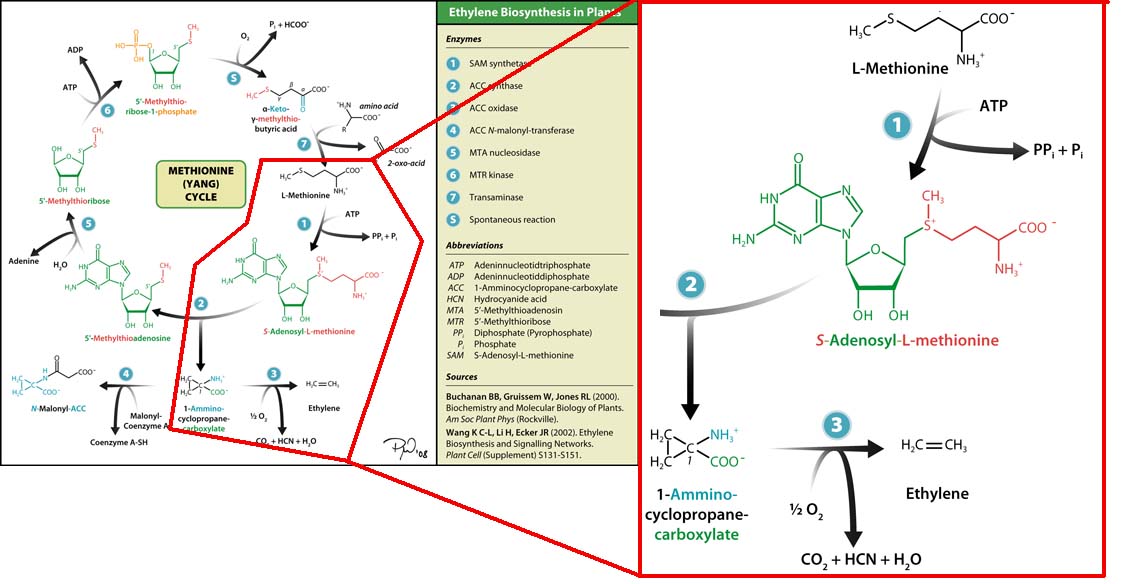
Plants synthesise ethylene naturally. Ethylene is a plant hormone, which can induce plants to grow and fruit to ripen. Ethylene biosynthesis occurs in three steps, starting with the conversion of the amino acid methionine to S-adenosyl-L-methionine (SAM) by the enzyme SAM synthase. SAM is then converted to 1-aminocyclopropane-1-carboxylic-acid (ACC) by the enzyme ACC synthase. The final step involves the action of the enzyme ACC-oxidase to oxidise ACC to ethylene.
So what are the more common items we can relate to?
A case study: plastic water bottles
Let’s explore example of an ethylene based product: disposable plastic water bottles. These bottles are made from Polyethylene Terephthalate (PET), and according to the Pacific Institute and the Bottled Water Alliance, over 15,000 tonnes of PET was used in packaging for bottled water in 2009-10 in Australia, The manufacture of one tonne of PET produces about three tonnes of Carbon Dioxide, equating to over 45,000 tonnes of CO2 released into the atmosphere per year due to these plastic bottles. To manufacture the PET, approximately 53 million litres of oil was used in 2009-10 in the production of bottles for water in Australia. These figures exclude impacts from the mining and transportation of crude materials.
On their own, those numbers are incredible, but when you take into account every other bottled product - soft drinks, fruit juice, sport drinks, milk, etc – it is clear that the manufacture of plastic bottles consumes an absolutely immense amount of energy and results in a tremendous amount of carbon pollution. Given modern society’s love affair with plastic bottles, policy-based mechanisms to discourage their use are unlikely to be effective. What could work is a technological solution, by which ethylene manufacture is decoupled from fossil fuels.
Our vision is to genetically engineer Escherichia coli to produce ethylene at room temperature, at low cost, with low energy reqirements and using renewable organic feedstocks.
(Sources: [http://www.pacinst.org/topics/water_and_sustainability/bottled_water/bottled_water_and_energy.html The Pacific Institute] and [http://www.bottledwateralliance.com/ The bottled Water Alliance])
Experimental plan
We attempted to use the plant ethylene biosynthesis pathway machinery to genetically engineer Escherichia coli to produce ethylene by the same pathway. The ultimate goal of the project is to remove the need for the high energy steam cracking process and decrease the reliance of fossil fuels to produce ethylene. The main benefit of our design is that once the ethylene is captured, it can be directly feed back into exhisting petrochemical infrastructure, therefore economically speaking there would not be a need for job losses through finding a new source of ethylene.
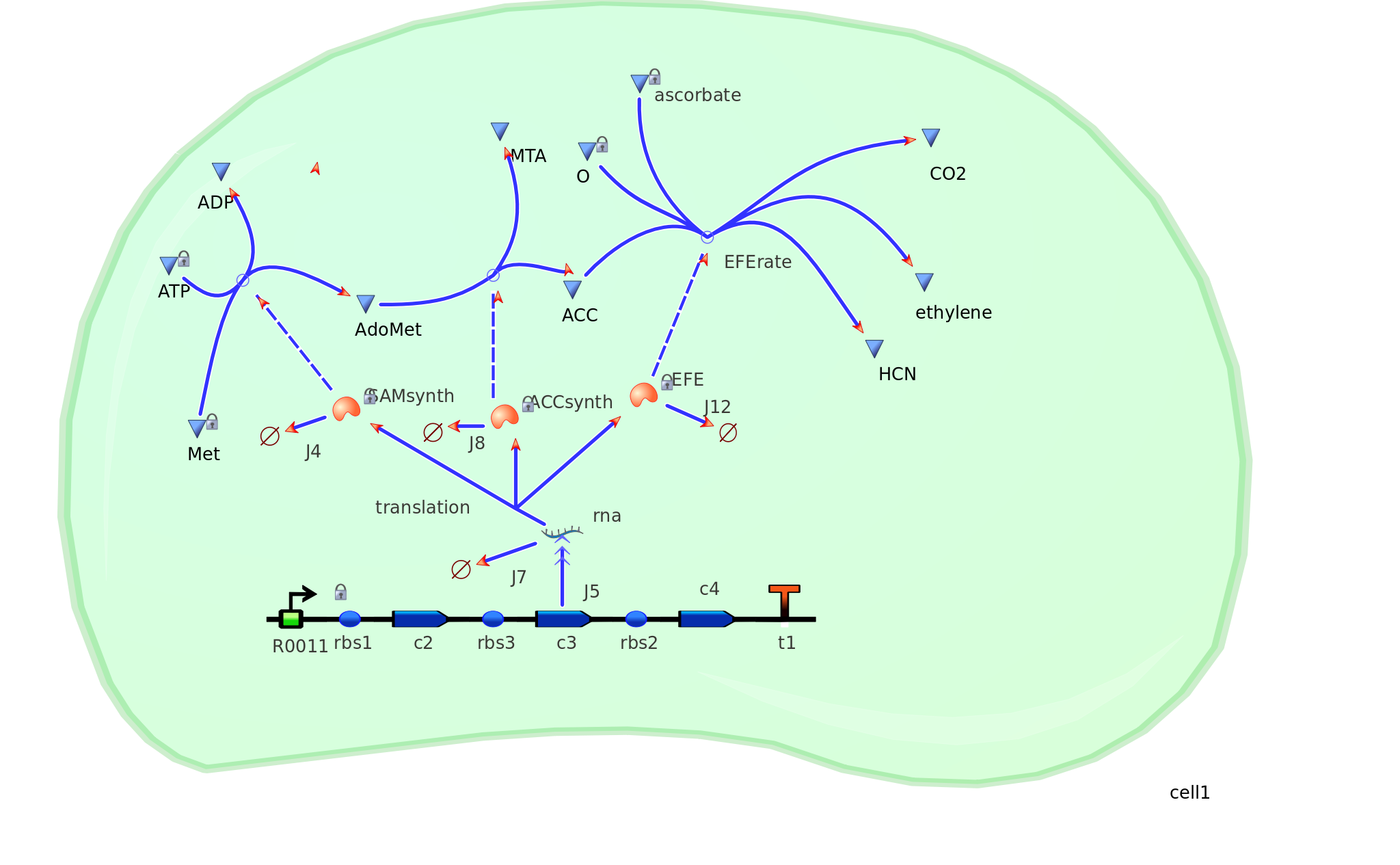
The Yang Cycle otherwise known as Methionine cycle is a biosynthesis cycle using methionine as a base molecule to produce several different products. We plan to clone three enzymes, SAM synthase, ACC synthase and ACC oxidase from apple and tomato plants and express them together in E. coli.
The three key enzymes we require are highlighted in the image, SAM synthase, ACC synthase and ACC oxidase. SAM synthase converts methionine into S-Adenosyl-L-Methionine (SAM), using ATP for an adensoyl group. The second step involves ACC synthase, which cleaves the amino butyrate from SAM, releasing 1-aminocyclopropane-1-carboxylic acid (ACC). Released ACC is then processed by ACC Oxidase which converts ACC to ethylene by cleaving the carboxylic acid off as carbon dioxide and its neighboring carbon with the amino group as cyanide gas. By using such a system to produce ethyene gas we can potentially reduce costs involved with current production methods by reducing temperature requirements by 30 fold.
For future iGEMers these biobricks can potentially be used for future project involving cellular signalling through ethylene production and ethylene receptors, which can be cloned from plants.
 "
"