Team:Monash Australia/Project
From 2010.igem.org

|
|
|||
| Overall project | Ethylene products we can relate to | In simpler terms | Experimental plan | Results |
Overall project
Monash University having a heavy movement to reduce the impact humans have on the planet has inspired our first iGEM project. After some initial research, we pondered on the concept of degrading plastics or cellulose into useable components. After discovering this has been a heavy focus by a number of different groups and past iGEM teams, so we then decidesd to look into producing some sort of useful product. After some investigation, we found that ethylene is a heavily used organic compound that is also naturally produced by plants. With our heavy reliance on this compound for plastics and in the food industry, we believe that it may be possible to develop a system that one day could be capable of replacing current production methods, which can reduce the impact of producing plastics has on the enviroment.
So what is ethylene used for?
Just about anywhere you go in the world you can find an ethylene based product. Just about everything you do today will have you come in contact or has come in contact with a ethylene based product. One of the most common ethylene based product is the water bottle. They come in all different shapes and sizes and can be found in every country in the world. Ethylene can be polymerised to create products such as; detergents, plasticisers, synthetic lubricants and additives, but also as co-monomers in the production of polyethylenes; Oxidised to create surfactants and detergents, and ethylene glycol; Halogenation and hydrohalogenation to produce products PVC, Polyvinylidene chloride and ethyl bromide; Alkylation to produce styrene; and itself used as a fuel source and to ripen fruit.
How do we make ethylene
To produce ethylene there is a huge requirement of energy, Ethylene is produced in the petrochemical industry by steam cracking. In this process, gaseous or light liquid hydrocarbons are heated to 750–950 °C, inducing numerous free radical reactions followed by immediate quench to stop these reactions (−157 °C). This process converts large hydrocarbons into smaller ones and introduces unsaturation. Ethylene is separated from the resulting complex mixture by repeated compression and distillation. The average ethtlene producing plant requires 34,000 kW cracked gas compressor, a 22,000 kW propylene compressor, and a 11,000 kW ethylene compressor, equating to a huge amount of energy required. Currently the main source of ethylene is from distilation of crude oil and natural gas, as well as catalytic steam cracking which precusors are again fossil fuels.
How do plants make ethylene
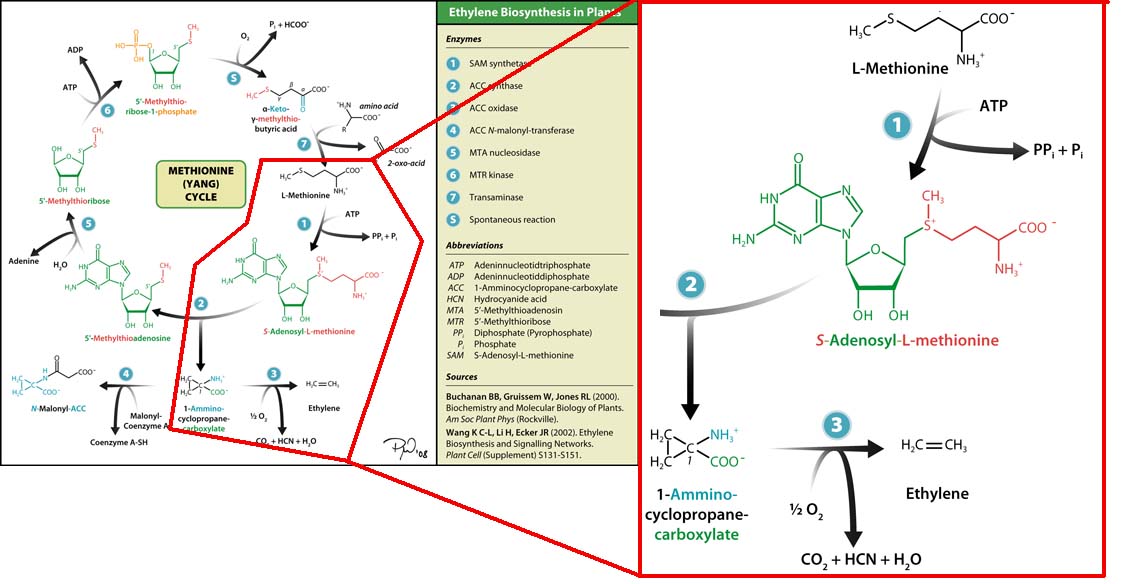
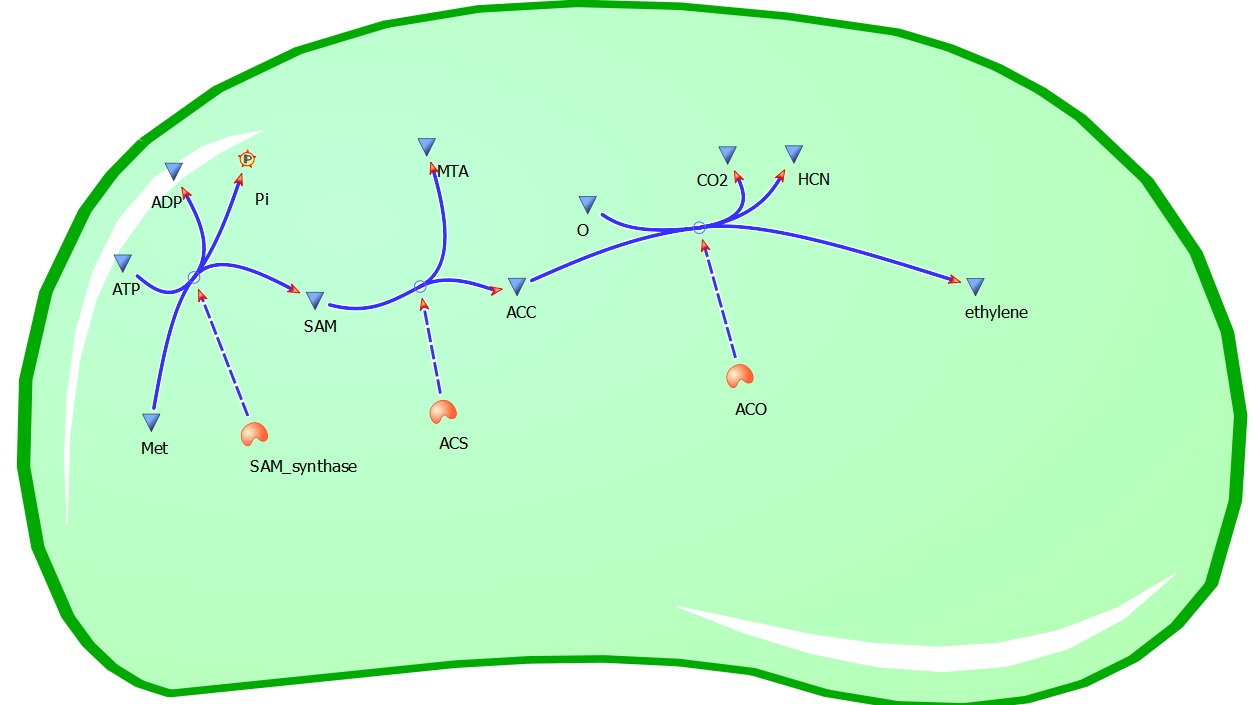
Plants also create ethylene. Ethylene is a plant hormone, which can induce plants to grow and fruit to ripen. in plants the biosynthesis of the ethylene occurs in three steps and starts with conversion of the amino acid methionine to S-adenosyl-L-methionine (SAM) by the enzyme SAM synthase. SAM is then converted to 1-aminocyclopropane-1-carboxylic-acid (ACC) by the enzyme ACC synthase. The final step involves the action of the enzyme ACC-oxidase to oxidiase ACC to ethylene.
So what are the more common items we can relate to?
In simplier terms
For something we can relate to, we can use the example of Bottled water. The polymer used to create bottle is Polyethylene Terephthalate (PET), and according to the Pacific Institute and bottled water alliance, over 15,000 tonnes of PET was used in packaging bottled water in 2009-10 in Australia, The manufacture of one tonne of PET produces about three tonnes of Carbon Dioxide, equating to over 45,000 tonnes of CO2 released into the atmosphere. To manufacture the PET, approximately 53 million litres of oil was used in 2009-10 in the production of bottles for water in Australia. These figures exclude mining and transportation of crude materials. These figures are only for bottled water, when you take into account every other bottled product; soft drinks, fruit juice, sport drinks, milk, etc there is a huge amount of energy and carbon dioxide produced. We can not completely stop the use of plastic bottles, in fact it is probably impossible to imagine life without plastic bottles, and we therefore were inspired to find a way to provide the precursor at a much lower impact to the planet. By genetically engineering Escherichia coli to produce ethylene, we remove the need and risks of mining and processing oil, and the extraction/production of ethylene which is a energy intensive and a heavily polluting process. We also decrease the reliance on mining oil which can lead to disastrous oil spills, such as the recent Gulf of Mexico incident, which can affect the livelihood of many families, the impact it has on the environment, economic loss, and the destruction of wildlife. (Sources: [http://www.pacinst.org/topics/water_and_sustainability/bottled_water/bottled_water_and_energy.html The Pacific Institute] and [http://www.bottledwateralliance.com/ The bottled Water Alliance])
Experimental plan
We attempted to use the plant ethylene biosynthesis pathway machinery to genetically engineer Escherichia coli to produce ethylene by the same pathway. The ultimate goal of the project is to remove the need for the high energy steam cracking process and decrease the reliance of fossil fuels to produce ethylene. The main benefit of our design is that once the ethylene is captured, it can be directly feed back into exhisting petrochemical infrastructure, therefore economically speaking there would not be a need for job losses through finding a new source of ethylene.
The Yang Cycle otherwise known as Methionine cycle is a biosynthesis cycle using methionine as a base molecule to produce several different products. We plan to clone three enzymes, SAM synthase, ACC synthase and ACC oxidase from apple and tomato plants and express them together in E. coli.The three key enzymes we require are highlighted in the image, SAM synthase, ACC synthase and ACC oxidase. SAM synthase converts methionine into S-Adenosyl-L-Methionine (SAM), using ATP for an adensoyl group. The second step involves ACC synthase, which cleaves the amino butyrate from SAM, releasing 1-aminocyclopropane-1-carboxylic acid (ACC). Released ACC is then processed by ACC Oxidase which converts ACC to ethylene by cleaving the carboxylic acid off as carbon dioxide and its neighboring carbon with the amino group as cyanide gas. By using such a system to produce ethyene gas we can potentially reduce costs involved with current production methods by reducing temperature requirements by 30 fold.
For future iGEMers these biobricks can potentially be used for future project involving cellular signalling through ethylene production and ethylene receptors, which can be cloned from plants.
 "
"