bioLOGICS: Logical RNA-Devices Enabling BioBrick-Network Formation
Vision
Until today, 13.628 biobrick sequences[1] have been submitted to partsregistry, thereof 102 reporter units, 12 signaling bricks and xx sensing parts.
Since there, people are trying to arrange these single biological building blocks in such a manner that allows producing special biotechnological products (metabolic engineering), developing biological sensory circuits (biosensors) and even giving microorganisms the ability to react on multiple environmental factors and serve both as disease indicator and drug. These examples and further promising ideas were implemented on previous iGEM-competitions.[2][3][4]
The idea of combining the outcome of several iGEM teams to construct a complex synthetic biological systems in vivo falls at the last hurdle. For example, it is a major challenge to create a system that uses several sensoring BioBricks from different iGEM-teams which in turn regulates reportering BioBricks from other various teams. The difficulties arise from the fact, that each team uses a different principle how to access and functionally connect the respectively used biobricks. In order to combine and fully take advantage of these promising projects, our vision is to develop an adapter that allows interconnecting any arbitrary biobricks on a functional level.
Generally speaking, the above descirbed adapter has to meet the following requirements:
- The adapter has to be compatible to as many BioBricks as possible. This objective will guarantee that a large number of BioBricks can be connected.
- The adapter has to be able to connect numerous BioBricks in parallel.
- Biological orthogonality:
- Interfering with cellular components has to be as low as possible in order to avoid unwanted and perturbing side effects.
- The adapter is supposed to not only associate different BioBricks, but to functionally connect BioBricks in a precisely determined manner (including operations such as AND/OR/NOT).
Several biological logic units, devices and circuits have been developed so far[5], but to our knowledge, none of them provides the opportunity to be a universal smallest unit like a transistor is in computational science. We investigated several hypothetically principles, and decided to focus our practical work on the development of a RNA-RNA interaction-based transistor by implementing two approaches:
- Designing a synthetic version of a RNA-sensitive riboswitch, which is based on the principle of shifting between a state of antitermination and termination as observed for attenuator operons in nature.

- Coupling this principle to the phenomenon of tiny abortive RNA´s, which cause antitermination in T7-termintors.

To evaluate functionality of our molecular switches, we developed several in vivo and in vitro assays and relied on existing assays.
Thus we don´t want to develop additional biobricks, we rather want to establish a platform to realize the full opportunity of the biobrick system. This would enable people easily setting up sensor - reporter circuits AND interconnect them to complete biological chips... the way to real artificial cells and synthetic biology. (zu krass?)
Erklärung für Konzept:
Both systems have to be tested for their ability of switching between termination and antitermination only in the presence of a signal. Therefore, our switches are generally divided into a switching unit, which is responsible for the functional shift between termination and antitermination, and a recognition unit, detecting the presence of a signal and defining specificity of our switch.
Reference
[1] http://partsregistry.org/cgi/partsdb/Statistics.cgi
[2] https://2009.igem.org/Team:Imperial_College_London/M1 encapsulation
[3] https://2009.igem.org/Team:TUDelft
[4] https://2008.igem.org/Team:Heidelberg
[5] Smolke and so on....
Although classical molecular biology and genetic engineering equipped the science community with many functional proteins and possible applications, using and linking different parts together to a working network still requires highly complicated strategies. The switches established in molecular biology, for example the lac operon, are highly limited, as most of them rely on interactions including metabolites and proteins providing only one on/off-signal. Thus, it is hardly possible to build up a logic network inside a cell without interferences between different switches. Our approach is to change this by developing a new and more robust way to control E. coli cells using RNA-RNA-interaction based switches, which we call bioLOGICS.
These switches allow an easy construction of networks consisting of AND/OR circuits. The major advantage of our RNA-based units is the possiblility to easily upscale and to include parameters for tailored protein expression control.
This is a major advantage towards ribozyme and especially protein based networks. While the complexicity of protein-protein interactions may work for cells, constructing networks without just copying complete operons is hardly possible. With the small size of our bioLOGICS, ten logic units occupy the space of an average protein sequence on a plasmid. Circuits based on bioLOGICS may play a key role for gene regulation with more variations than just on/off in the future.
Concept
The basic principle of our switches are short RNA sequences, the scientific idea shares similiarities with the principle of antitermination but also inherits a completely new way of RNA based transcription regulation. We used three-dimensional structure predictions and thermodynamic calculation to develop a set of switches - about 50 nucleotides - and signals - about 20 nucleotides. The switch forms a stem loop causing transcription termination, which can be resolved upon binding of a signal. On/off switching can therefore be easily controlled by signal avaiability and provides a new concept to control gene transcription.
Read more
Our switches are based on the principle of transcriptional antitermination. Transcription can be cancelled by the formation of a RNA stem loop in the nascent RNA-chain, which then causes the RNA polymerase to stop transcription and fall off the DNA. We use sequences as transcriptional switches, that are calculated to be capable of stem loop formation.
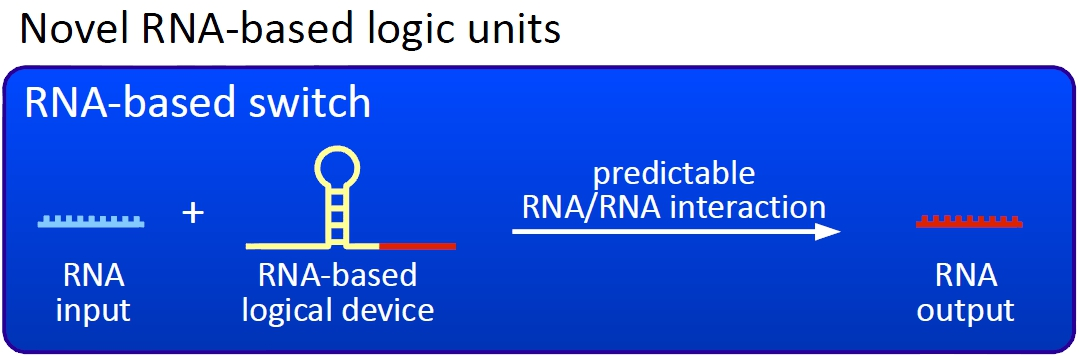
Novel switches based on RNA-RNA interaction
We plan to control the termination at these switches with a small RNA molecule (our RNA "signal") that is complementary to a part of the stem loop forming sequence. This small functional RNA inhibits the stem loop formation by complementary base-pairing and hence avoids transcription termination. The signal is composed of two parts: While the first part provides specifity (recognition site), the second part causing stem loop disintegration (functional core) can in principle be the same for all bioLOGICS. Therefore variation of the first part allows the construction of an endless number of switches. The functional core causing stem loop disintegration is based on a working system (see attenuation) established by nature. Different stem loops were tested in this effort: Regulatory parts from the E. coli trp-operon, his-operon and one based on previous iGEM-work.
The initial signal can be provided by various metabolic compounds and stimuli and the output signal can be anything DNA-coded, too. In the last years, many working sensory systems were submitted to the Partsregistry. Those parts can now be utilized as inputs for our network. BioLOGICS provides a new way to use the whole potential of iGEM distributions connecting different parts for totally new applications.
The major advantage over conventional protein based devices and even riboswitches is the small size of our basic units, its easy construction, huge variability and easy upscaling to complex networks in cells. Furthermore introduction of an RNA network into E. coli is especially easy - all informations can be coded on one plasmid. In comparison to protein networks, the elegance of RNA based networks lies in its functionality, simplicity and predictability. Since RNA-RNA interactions are highly predictable and since our system is based on the variation of one principle in many switches, simulations of our networks are much more accurate than those of comparable systems currently available. Incorporation of established Biobricks allows a high diversity of input and output signals providing a whole new level of cell control.
Close
Network construction
Designing complex biological networks based on either traditional protein engineering or our new bioLOGICS is still a complex task. We developed a software which allows the fast construction of a bioLOGICS based networks.
To read more about this, look at our Software page
Work Progress
Every network starts with a basic unit. While our declared aim is to enable networks allowing fine-tuning of gene expression beyond the regular on/off, exploring such an on/off switch/signal pair is the first step towards a functional network. We constructed several units and tested their efficiency, robustness and reproducibility in vivo, in vitro and in silico. Furthermore we developed a software which allows easy constructions of networks delivering a ready network. Conclusive elaboration of a few first RNA-based logic units is the major contribution of our iGEM team.
Results
 "
"











