Team:Freiburg Bioware/NoteBook/Labjournal/Cellculture
From 2010.igem.org
(→30.6 Examination of different Transduction methods) |
(→3.7 Seeding AAV293 for transfection with different plasmid amounts and harvesting methods) |
||
| Line 188: | Line 188: | ||
<br /> | <br /> | ||
<b>The motivation</b><br /> | <b>The motivation</b><br /> | ||
| - | + | ||
We want to check if the amount of plasmids is the reason for the low transduction efficiency. Our main problem is that the flow cytometry (FACS) is not available, so the results have to be checked via fuorescence microscopy. | We want to check if the amount of plasmids is the reason for the low transduction efficiency. Our main problem is that the flow cytometry (FACS) is not available, so the results have to be checked via fuorescence microscopy. | ||
| - | <br /> | + | <br /><br /> |
<b>The plan</b><br /> | <b>The plan</b><br /> | ||
<br /> | <br /> | ||
Revision as of 21:33, 9 October 2010
Team:Freiburg Bioware/SubNoteBook
- March (labday 1)
- April (labday 2 - 5)
- May (labday 6 - 17)
- June (labday 18 - 45)
- July (labday 46 - 75)
- August part 1 (labday 76 - 92)
- August part 2 (labday 93 - 106)
- September part 1 (labday 107 - 123)
- September part 2 (labday 124 - 135)
- October part 1 (labday 136 - 145 )
- October part 2 (labday 146 - 155 )
- October part 3 (labday 156 - 166 )
- November (labday 167 - 170 )
- Cellculture
Cellculture
This subpage is for cellculture, the structure of each experiment is following:
Date of first action, title of the experiment
The motivation
This short introduction provides an inside into our thoughts for this experiment.
The plan
In this section, the design of the experiment is explained.
The results
Interpretation and discussion of our results.
14.6 Seeding AAV293 for transfection
The motivation
We want to create viral stocks with the stratagene R/C, the gene of interest is mVenus. This is a qualitative control.
The plan
AAV 293 and HT1080 Cells have been splitted and plated out on 10 cm dishes for transfektion.
The Cells were accounted by using the Neubauer-Meteringchamber.
After harvesting by following the standard protocol the pallet have been resuspendiated in 15ml of DTT medium.
2,5µl of this cell suspension have been mixed with 47.5µl of trypan blue.
We counted 12,5x 10^6 cells/ml for the AAV293 cell line and 10x10^6 cells /ml for the HT1080 cell line.
The AAV 293 cells have been plated out on four dishes with 250µl of the cell suspension, on one plated with 1ml and on another dish with 1.5ml. note that that the date is wrong on the plates and the flasks! Cells have been already plated out on the 14th. of June.
We also seated two flasks one for each cell line. Therefor we used 1ml of the HT1080 cell suspension and two ml of the the AAV293 cells.
1)
- pAAV_iGEM_mVenus_YFP (glycerol stock B34, P39) P39 has the correct sequence (confirmed)
- pHelper
- pAAV_RC
2)
- pAAV_iGEM_mVenus_YFP (glycerol stock B33, P38) P38 (we dont know if P39 has the correct sequence!)was used because the amount of P39 was not sufficient enough for four Transfections!
- pHelper
- pAAV_RC
Plasmid concentrations:
pAAV_RC: 1 µg/µl
pHelper: 280 ng/µl
pAAV_iGEM_mVenus_YFP, P39: 180,83 ng/µl
pAAV_iGEM_mVenus_YFP, P38: 179,75 ng/µl
Cellculture clone 1 and 2 were transfected with P39. Cellculture clone 3 and 4 were transfected with P38.
We could only pipet 3,3 µg (instead of 10 µg) from each of the 3 plasmids into the 15 ml falcons due to insufficient amount of plasmid.
Adrian tried to examine the precipation of the CaCl2+ DNA Clusters. We have to optimize the pH of our 2xHBS at the moment it is 11.12.
The viral stocks are harvested according to standard protocol.
The results
According to the stratagene protocol, transduction efficencies about ~80% are achievable. We performed every step under guidance, so probably something is wrong with the buffers, cells or even the standard protocol. The next step is to repeat this transfection.
19.6 Seeding AAV293 for second transfection according to standard protocol
The motivation
We want to repeat the last transfection to exclude faults in practical
The plan
Six transfections were performed according to the standart protocol: Media:Freiburg10_Transfection_protocoll.pdf
Three 10 cm cellcluture dishes with 293 cells were transfected with 10 µg DNA (3,33 µg each plasmid) and the other three 10 cm cellculture dishes with 30 µg DNA (10 µg DNA each plasmid).
Plasmids:
- pAAV_iGEM-MCS_mVenus (P41), 507 ng/µl
- pAAV_RC , 1000 ng/µl
- pHelper , 280 ng/µl
- Transduction of 2x6 wells was successfully done (14.25)
- one six well was transduced with 10µg the other with 30µg DNA amount
The results
The Transduction efficency is still quite low! We have to optimize the stratagene protocol!
30.6 Examination of different Transduction methods
The motivation
We want to rise the amount of transduced particels, instead of 500µl, 1000µl of the AAV-stock getting pipetted into each well. the second plate gets stimulated via UV-light to boost the expression of DNA-Repair mechanisms. This should enhance the second strand synthesis of our ssDNA-GOI => a rise of YFP Expression!
The plan
1. Plate I(A is up)
| 3x10^5 cells | 3x10^5 cells | 3x10^5 cells |
| 3x10^5 cells | 3x10^5 cells | 3x10^5 cells |
Transduction:
| 1000 µl of Viral Stock DROPPED | 1000 µl of Viral Stock gently resuspended | 1500 µl of Viral Stock hard resuspending |
| 1000 µl of Viral Stock DROPPED | 1000 µl of Viral Stock gently resuspended | no Transduction |
2. Plate II (A is up)
| about 3x10^5 cells | only medium | only medium |
| about 3x10^5 cells | only medium | only medium |
Transduction:
| 1500 µl of Viral Stock gently resuspended | no Transduction | no Transduction |
| 1500 µl of Viral Stock gently resuspended | no Transduction | no Transduction |
The results
All cells are dead. It was not possible to take pictures because the dead cells were all clumped up.
Looks like we should not transduce 3x10^5 cells with 1000 µl (or more) of our viral solution because almost all cells died in all approaches and the medium indicator was yellow.
So we can not say if resuspendig the cells with the viral soultion is a useful alteration of the standard protocol.
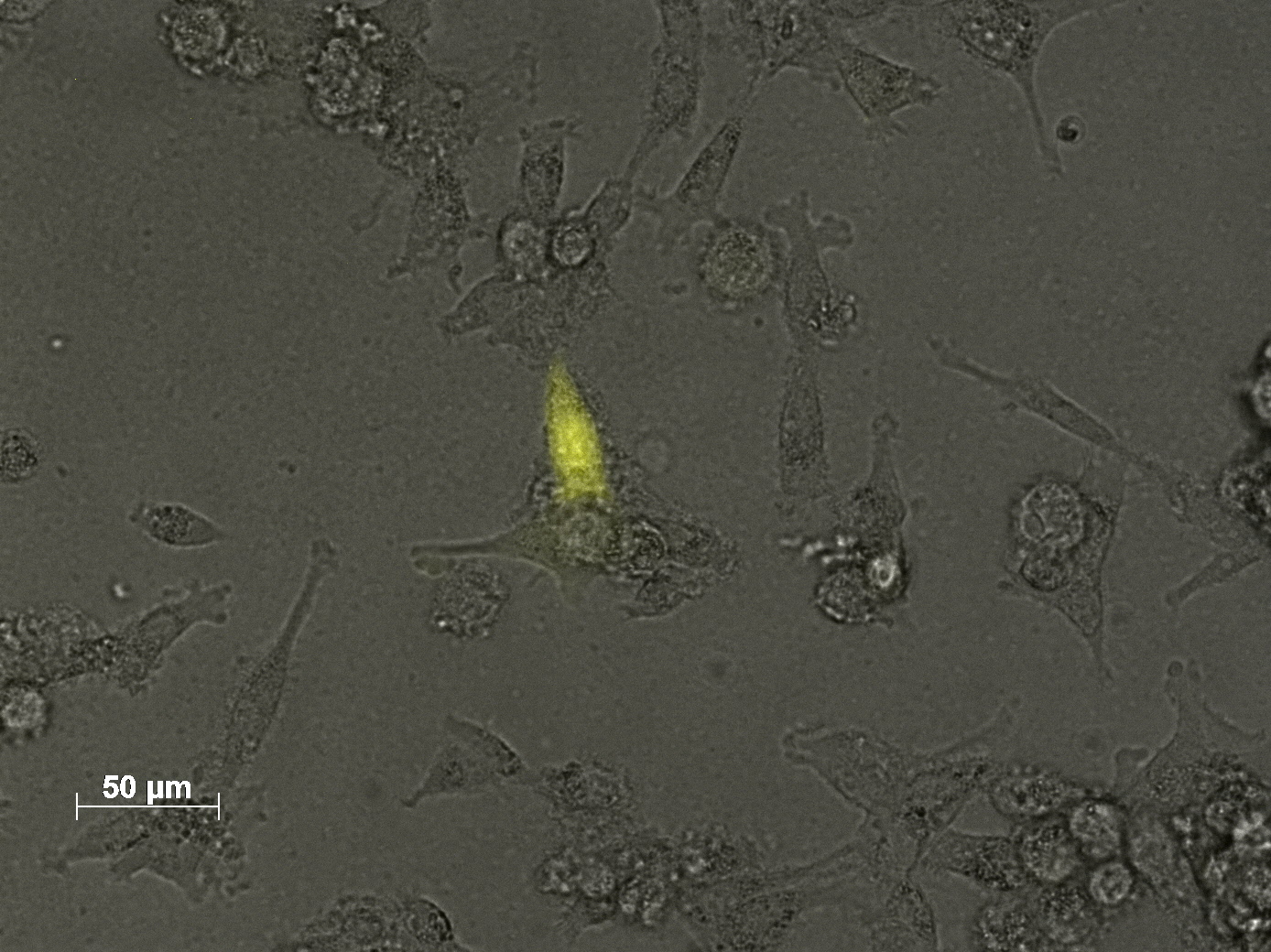
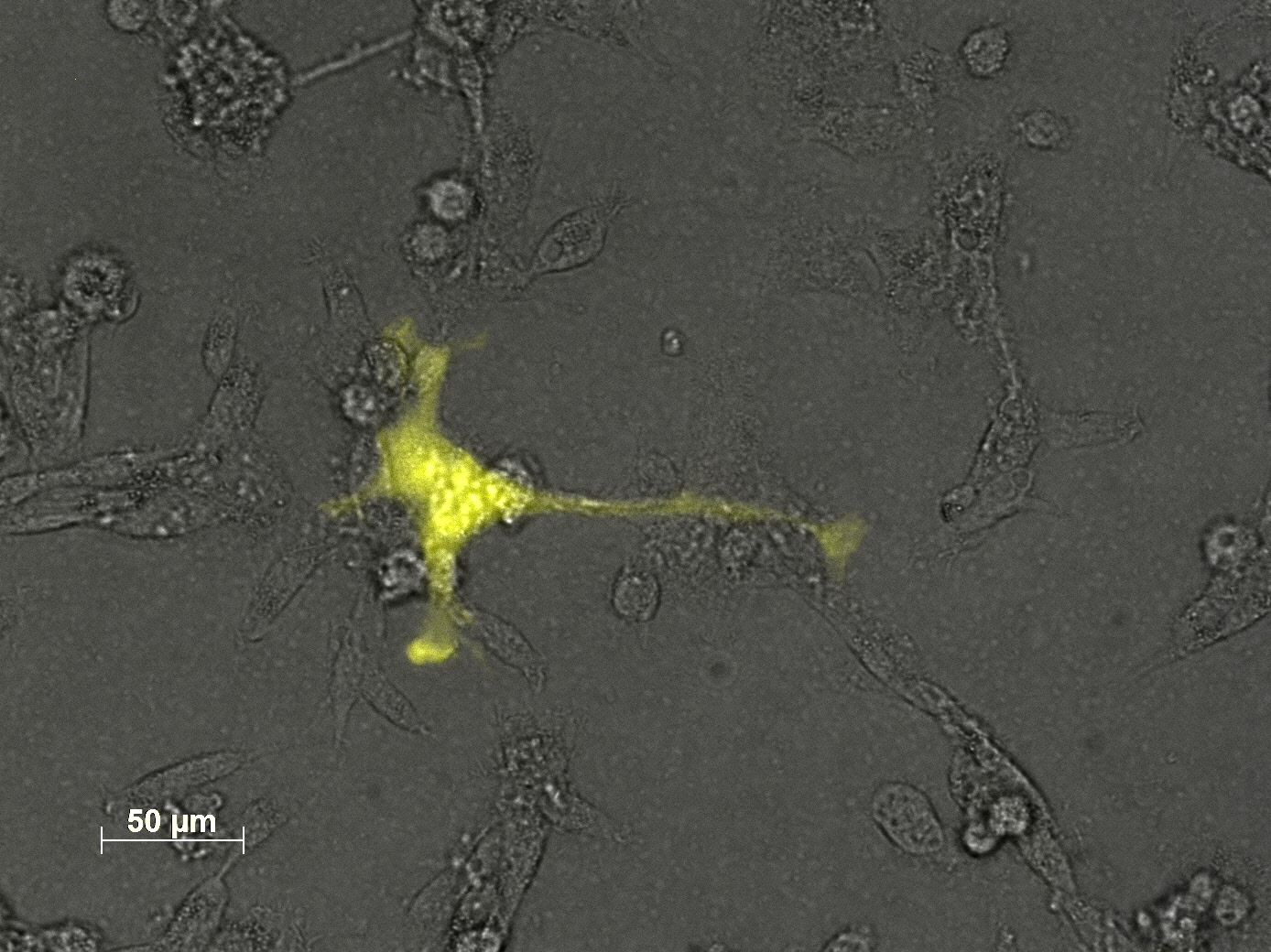
3.7 Seeding AAV293 for transfection with different plasmid amounts and harvesting methods
The motivation
We want to check if the amount of plasmids is the reason for the low transduction efficiency. Our main problem is that the flow cytometry (FACS) is not available, so the results have to be checked via fuorescence microscopy.
The plan
Transfection with P41 was performed according standart protocol.
Two transfections where carried out with 10µg (3,33 µg each plasmid) DNA and the other two with 20µg DNA (6,66 µg each plasmid).
We want to investigate which of the following approaches yields a better transduction efficiency.
- Half of the transfected cells were exposed 3 cycles of thawing and freezing and then centrifuged (15 ml falcon, 2100G). The supernatant was transfered into a new 15 ml falcon and used for transduction.
- The other half was centrifuged at 300 G for 5min . The supernatant was transfered into another 15 ml falcon and the pellet was resuspended with 5 ml of DMEM. The content of these two falcons was also exposed to 3 cycles of thawing and freezing and then used for transduction.
- We have got 2 10 cm cellculture dishes with 10µg and 2 dishes with 20µg DNA used for transfection.
Approach with standart protocol (one dish with 10 and the other with 20µg Plasmids)(2100 G and 3 cycles of freezing and thawing)
Deviations from the standart protocol:
- The cells were centrifuged at 2100 G instead of 10.000 G
- The viruses were harvested after 44 hours
- 3 cycles of freezing and thawing
- used plasmids: 10µg, 20µg YFP
- we have 10µg and 20µg from standart protocol => SV
- we have 10µg and 20µg from standart protocol pellet => Pellet
- we have 10µg and 20µg from standart protocol suspension => Super
- amount of 500µl
1. Plate I(A is up)
| 10µg dropped Super | 10µg dropped Super gently resuspending | 10µg dropped Super gently resuspending |
| 10µg Super gently resuspending Super | 20µg dropped Super gently resuspending 1:10 dilution | control |
2. Plate I(A is up)
| 10µg dropped Super | 10µg dropped Super gently resuspending | 10µg dropped Super gently resuspending |
| 10µg Super gently resuspending Super | 20µg dropped Super gently resuspending 1:10 dilution | control |
3. Plate I(A is up)
| 10µg dropped Pellet | 10µg dropped Pellet gently resuspending | 10µg dropped Pellet gently resuspending |
| 10µg Super gently resuspending Pellet | 20µg dropped Pellet gently resuspending 1:10 dilution | control |
The results
We only took some pictures from single cells, because there was no significant difference in fluorescence between each sample.
The main conclusion of this experiment is, that we have to get the flow cytometry started.
4.7 Transfection with different amounts of plasmids
The motivation
We want to check if the mVenus expression/ transduction efficiency can be extended with higher amounts of DNA.
The plan
The Transfection was performed with 10, 18 and 24 µg per plasmid (mVenus, pHelper and R/C).
the harvest was performed according to standard protocol:
- 3 Plates got transduced
1. Plate I(A is up) completely 10 µg
| 300µl dropped | 300µl resuspended | 450µl resuspended |
| 600µl dropped | 600µl resuspended | control |
2. Plate II(A is up)completely 18 µg
| 300µl dropped | 300µl resuspended | 450µl resuspended |
| 600µl dropped | 600µl resuspended | control |
3. Plate III(A is up) completely 24 µg
| 300µl dropped | 300µl resuspended | 450µl resuspended |
| 600µl dropped | 600µl resuspended | control |
The results
FACS analysis of different treated HT1080 cells was done (18 samples)
The first percentage refers to the living and fluorescent cells and the second percentage to the total amout of living cells.
The data are listed this way:
1. Plate I(A is up)
| A1 | A2 | A3 |
| B1 | B2 | B3 |
1= Plate 1 A1 : 0% YFP-positive Cells (there were only a few cells in this sample)
2= Plate 1 A2 : 27,7% YFP-positve Cells from 73,3%
3= Plate 1 A3 : 17,3% " from 63,1%
4= Plate 2 A1 : 32,9% " from 75,3%
5= Plate 2 A2 : 27,2% " from 76,5%
6= Plate 2 A3 : 30,7% " from 73,4%
7= Plate 3 A1 : 26,8% " from 76,0%
8= Plate 3 A2 : 30,5% " from 74,2%
9= Plate 3 A3 : 30,7% " from 75,2%
10= Plate 1 B1 : 6,25% " from 36,1%
11= Plate 1 B2 : 6,42% " from 68,4%
12= Plate 1 B3 : 0,05% " from 89,5%
13= Plate 2 B1 : 20,5% " from 72,5%
14= Plate 2 B2 : 15,7% " from 73,6%
15= Plate 2 B3 : 0,02% " from 85,4%
16= Plate 3 B1 : 19,5% " from 73,3%
17= Plate 3 B2 : 20,8% " from 75,7%
18= Plate 3 B3 : 0,07% " from 84,8%
The calculated value written into the table describes the fluorescent amount of cells from the living cells. Example: 1 A2 : 27,7% YFP-positve cells of 73,3% living cells : 27,7/73,3 = 0,378
Conclusions
- Higher amounts of Plasmids (24, 30µg) seems to have no obvious effect on YFP expression
- Theres no difference in YFP expression comparing resuspended samples to not resuspended samples
- The amount of dead HT cells is about 25%, it is not clear if it is either AAV induced or mechanic stress (Trypsin, washing procedure)
Next steps:
- does the amount of GOI (ng plasmids) has an effect on YFP expression
- compare transduced to non transduced cells (vitality), or in other words is the mechanic stress responsible for 25% dead cell ratio?
- comparison of transduction efficiency of different virus stock production routines
14.7 Seeding AAV293 for TKGMK constructs
The motivation
We want to create the first viral particles with TKGMK as gene of interest.
The plan
30µg Plasmid were used (pHelper + pAAV_RC+ pAAV_RFC25_mgmk_TK30)
VIRUS no GOI :pHelper + pAAV_RC
VIRUS 1 :pHelper + pAAV_RC+ pAAV_RFC25_mgmk_TK30 clone 1 : P54
VIRUS 2 :pHelper + pAAV_RC+ pAAV_RFC25_mgmk_TK30 clone 2 : P55
VIRUS 3 :pHelper + pAAV_RC+ pAAV_RFC25_mgmk_TK30 clone 3 : P56
The results
Unfortunately the stocks never were used.
7.8 Seeding AAV293 for TKGMK and mVenus stocks
The motivation
First we want to create new stock of AAV without GOI, the next viral stock is with correct TK/GMK, the last stocks are with different YFP-amounts to check if the amount of GOI is a critical step for transduction effency (transgene expression)..
The plan
| Plate | pAAV_RC/µg | pHelper/µg | GOI/µg |
| 1 | 3,3 | 3,3 | no GOI |
| 2 | 6,6 | 3,3 | no GOI |
| 3 | 3,3 | 3,3 | 3,3 TK_GMK clone1 |
| 4 | 3,3 | 3,3 | 3,3 TK_GMK clone1 |
| 5 | 3,3 | 3,3 | 3,3 TK_GMK clone2 |
| 6 | 3,3 | 3,3 | 3,3 YFP |
| 7 | 3,3 | 3,3 | 10 YFP |
| 8 | 3,3 | 3,3 | 20 YFP |
| 9 | 3,3 | 3,3 | 40 YFP |
| 10 | 3,3 | 3,3 | 60 YFP |
transduction was performed according to standard protocol.
Platte 1:
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus 1 | 300µl virus 2 |
| B | control, no virus | 600µl virus 1 | 600µl virus 2 |
Platte 2:
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus 3 | 300µl virus 4 |
| B | control, no virus | 600µl virus 3 | 600µl virus 4 |
Platte 3:
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus 5 | 300µl virus 6 |
| B | control, no virus | 600µl virus 5 | 600µl virus 6 |
Platte 4:
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus 7 | 300µl virus 8 |
| B | control, no virus | 600µl virus 7 | 600µl virus 8 |
Platte 5:
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus 9 | 300µl virus 10 |
| B | control, no virus | 600µl virus 9 | 600µl virus 10 |
Platte 6:
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus 3 | 300µl virus 4 |
| B | control, no virus | 600µl virus 3 | 600µl virus 4 |
Platte 7:
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus 3 | 300µl virus 4 |
| B | control, no virus | 500µl virus 3 | 500µl virus 4 |
Platte 8:
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus 5 | 300µl virus 5 |
| B | control, no virus | 600µl virus 5 | 600µl virus 5 |
The test for functionality for TK GMK was done with the Prodrug Cymeven ganciclovir 500mg from Roche. The final concentration was 0,05 mM in the wells. After two days pictures were taken.
The results
As you can see, the TKGMK cell killing mechanism works! The samples with targeted mVenus constructs couldnt be checked because the FACS machine was not working. The fluorescence was checked with microscopy, the transduction efficiency is still low.
11.8 Transduction of HT1080 with no AAV, AAV without GOI and AAV with 10µg
The motivation: In the past, the percentage of living cells in FACS was very low (~ 70%) so we want to check if the AAV-induced stress is responsible for cellular death, or the detaching procedure (trypsin and mechanical stress).
The plan
Plate I
| control, no cells | no GOI 150µl | 10µg 500µl |
| control, no virus | no GOI 300µl | 10µg 750µl |
Plate II
| control, no cells | no GOI 150µl | 10µg 500µl |
| control, no virus | no GOI 300µl | 10µg 750µl |
Plate III
| control, no cells | no GOI 150µl | 10µg 500µl |
| control, no virus | no virus | 10µg 750µl |
The results The YFP expression is still quite low. This was kind of anticipated, the success in this experiment is the fact,that the amount of living cells is at a very high level! (~90%). In future preparations of FACS samples use cold PBS!
14.8 Seeding different amounts of AAV293 for transfection
The motivation
We want to know which confluence of AAV293 cells is optimal for AAV production.
The plan
We seed different amounts of cells in 10 cm2, and check the highest titer.
| Plate | amount of cells |
| 1 | 100 000 |
| 2 | 200 000 |
| 3 | 400 000 |
| 4 | 500 000 |
| 5 | 800 000 |
| 6 | 1 000 000 |
| 7 | 1 200 000 |
| 8 | 1 500 000 |
| 9 | 1 750 000 |
| 10 | 1 750 000 |
The cells were transfected with 40µg YFP, 3,3µg Rep/Cap and 3,3µg pHelper.
Transduction was performed according to standard protocol.
Plate 1 YFP: 200.000 cells per well
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 165µl virus (1) | 165µl virus (2) |
| B | control, no virus | 165µl virus (1) | 165µl virus (2) |
Plate 2 YFP: 200.000 cells per well
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 165µl virus (3) | 165µl virus (4) |
| B | control, no virus | 165µl virus (3) | 165µl virus (4) |
Plate 3 YFP: 200.000 cells per well
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 165µl virus (5) | 165µl virus (6) |
| B | control, no virus | 165µl virus (5) | 165µl virus (6) |
Plate 4 YFP: 200.000 cells per well
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 165µl virus (7) | 165µl virus (8) |
| B | control, no virus | 165µl virus (7) | 165µl virus (8) |
Plate 5 YFP: 200.000 cells per well
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 165µl virus (9) | 165µl virus (10) |
| B | control, no virus | 165µl virus (9) | 165µl virus (10) |
17.8 transduction with viral stocks 20 µg and 40 µg
| A | control no cells | 300 µl virus (40 µg YFP) | 300 µl virus (20 µg YFP) |
|---|---|---|---|
| B | control no virus | 500µl P38 (pAAV2_mVenus) | 1000µl P38 (pAAV2_mVenus) |
| A | control no cells | 300 µl virus (40 µg YFP) | 300 µl virus (20 µg YFP) |
|---|---|---|---|
| B | control no virus | 500µl P38 (pAAV2_mVenus) | 1000µl P38 (pAAV2_mVenus) |
| A | control no cells | 300 µl virus (40 µg YFP) | 300 µl virus (20 µg YFP) |
|---|---|---|---|
| B | control no virus | 500µl P38 (pAAV2_mVenus) | 1000µl P38 (pAAV2_mVenus) |
| A | control no cells | 300 µl virus (40 µg YFP) | 300 µl virus (20 µg YFP) |
|---|---|---|---|
| B | control no virus | 500µl P38 (pAAV2_mVenus) | 1000µl P38 (pAAV2_mVenus) |
| A | control no cells | 150 µl virus (TKGMK clone 1) | 180 µl virus (TKGMK clone 1) |
|---|---|---|---|
| B | control no virus | 200 µl (TKGMK clone2) | 200µl (no GOI) |
16.8 Seeding AAV293 for transfection with new R/C
The motivation
We want examine the functionality of different R/C constructs and the new eGFP gene of interest. This transfection was performed with new cells which were thawed.
The plan
- 2x106 cells were seeded (instead of 3x106) --> hopefully better virus-production
- used plasmids:
- P50 c= 429 ng/µl (RC)
- P64 c= 500 ng/µl (pHelper)
- P229 c= 162 ng/µl (GOI=YFP)
- P228 c= 540 ng/µl (GOI=eGFP)
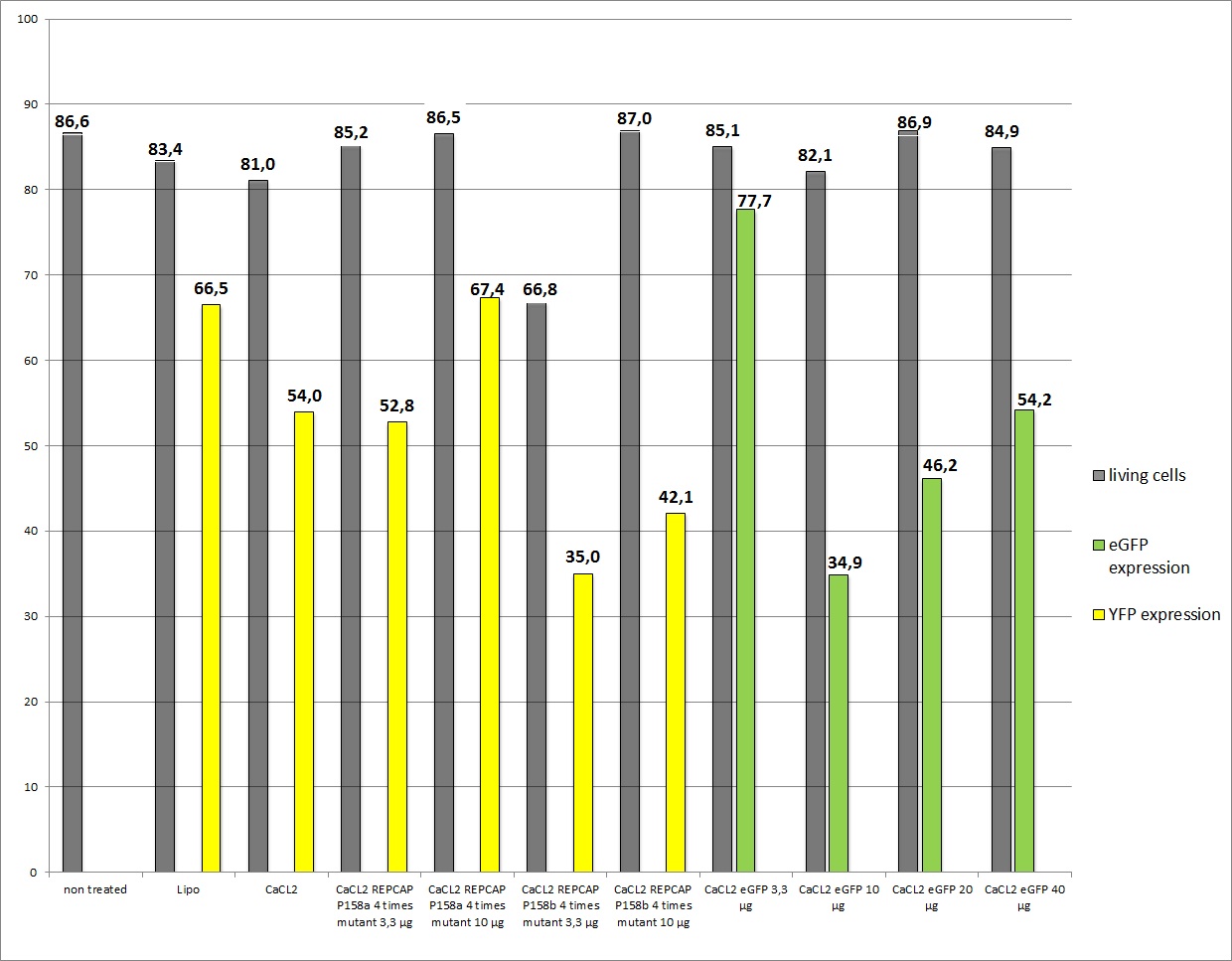
- P158a c= 1800ng/µl (RC mutant)
- P158a c= 1770ng/µl (RC mutant)
The transfection was performed according to standard protocol.
- 1. plate: Lipo-Transfection
- 2. plate: Lipo-Transfection
- 3. plate: 3,3 µg (RC), 3,3 µg (pHelper), 3,3 µg (YFP)
- 4. plate: 10 µg (RC), 10 µg (pHelper), 3,3 µg (YFP)
- 5. plate: 10 µg (RC mutant P158a), 10 µg (pHelper), 3,3 µg (YFP)
- 6. plate: 10 µg (RC mutant P158b), 10 µg (pHelper), 3,3 µg (YFP)
- 7. plate: 3,3 µg (RC mutant P158a), 3,3 µg (pHelper), 3,3 µg (YFP)
- 8. plate: 3,3 µg (RC mutant P158b), 3,3 µg (pHelper), 3,3 µg (YFP)
- 9. plate: 10 µg (RC), 10 µg (pHelper), 3,3 µg (eGFP)
- 10. plate: 10 µg (RC), 10 µg (pHelper), 10 µg (eGFP)
- 11. plate: 10 µg (RC), 10 µg (pHelper), 20 µg (eGFP)
- 12. plate: 10 µg (RC), 10 µg (pHelper), 40 µg (eGFP)
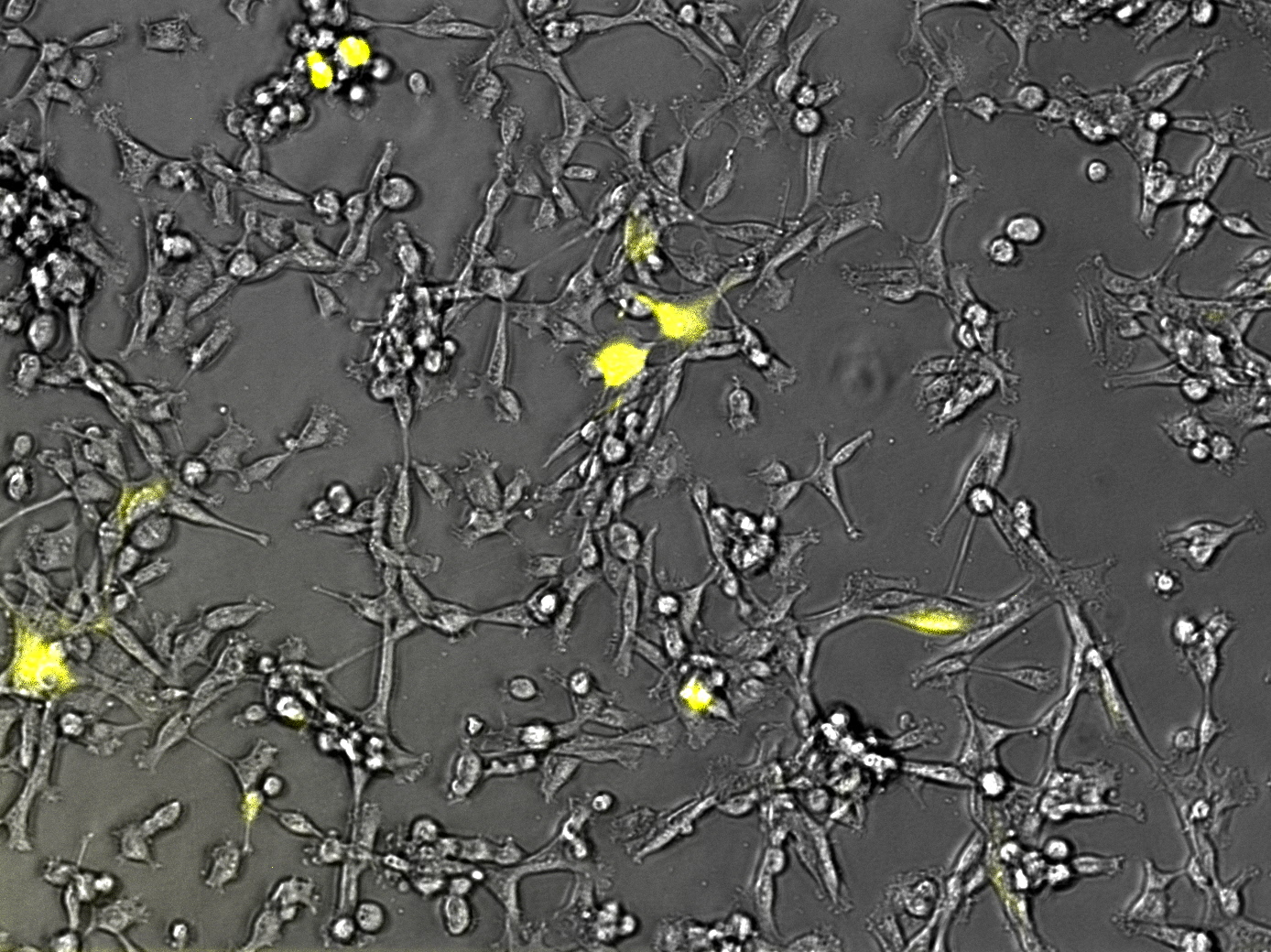
The results
As you can see the eGFP constructs very well. And even the transduction efficiency of the standard R/C is doubled.
20.8
The Motivation
Seeding HT1080 for transfection with viral stocks from 18.8
The Plan
We harvest four T75 flasks and seed them into 6er dishes (200.000 cells in each well)
the Results
The Facs data at 22.8
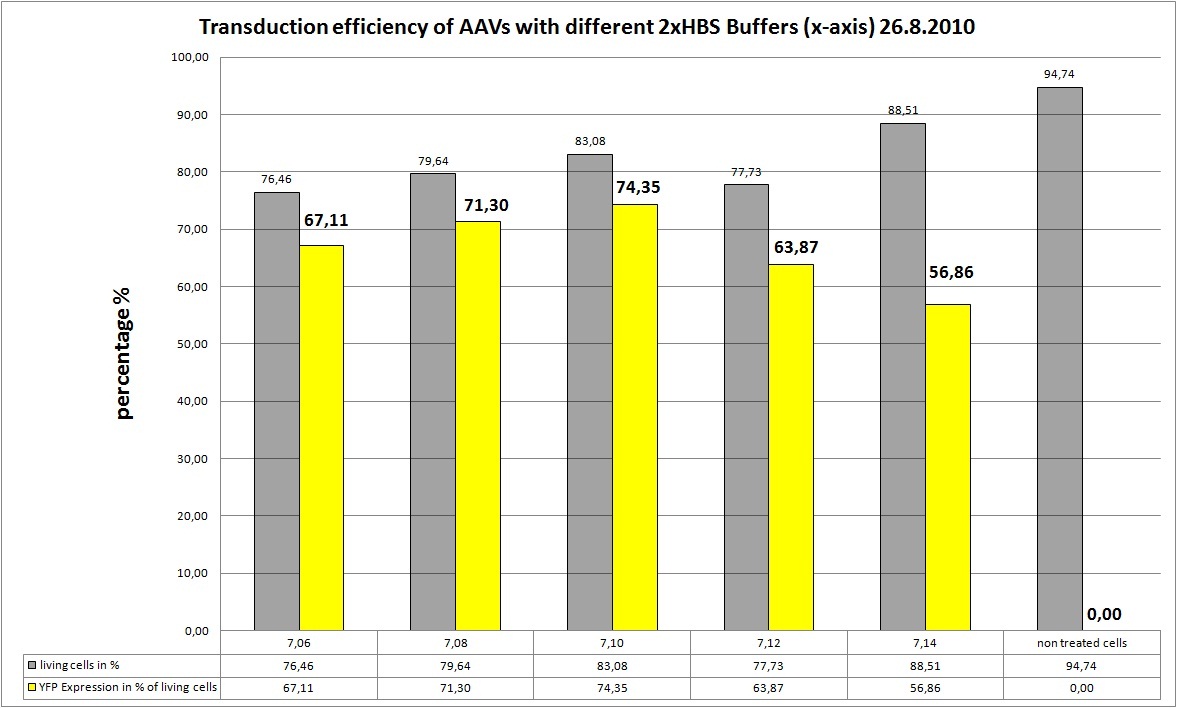
19.8 Seeding AAv293 for transfection with different HBS buffers
The Motivation
We want to try different 2xHBS Buffers (pH) and their influence on transfection efficiency!
The Plan
We try 5 different 2xHBS buffers:
- pH: 7.06
- pH: 7.08
- pH: 7.10
- pH: 7.12
- pH: 7.14
Used Plasmids: Standard protocol: 10µg of each plasmid
- GOI: mVENUS => P262, conc:1148ng/µl; used amount: 8,71µl
- GOI: TKGMK => P82b, conc:1500ng/µl; used amount: 6,67µl
- pHELPER => P64, conc:503ng/µl; used amount: 20µl
- RepCap => 10.8: 1348ng/µl; used amount: 7,41µl
Eight new viral stocks were made with pHelper:20µl and RepCap:7,41µl each:
- mVenus:8,71µl; pH of 2xHBS:7,06
- mVenus:8,71µl; pH of 2xHBS:7,08
- mVenus:8,71µl; pH of 2xHBS:7,10
- mVenus:8,71µl; pH of 2xHBS:7,12
- mVenus:8,71µl; pH of 2xHBS:7,14
- TKGMK:6,67µl; pH of 2xHBS:7,08
- TKGMK:6,67µl; pH of 2xHBS:7,10
- TKGMK:6,67µl; pH of 2xHBS:7,12
- Transduction of three 6-well plates:
Plate 1 YFP: 150.000 cells per well
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 500µl virus (1) | 500µl virus (2) |
| B | control, no virus | 500µl virus (1) | 500µl virus (2) |
Plate 2 YFP: 150.000 cells per well
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 500µl virus (3) | 500µl virus (4) |
| B | control, no virus | 500µl virus (3) | 500µl virus (4) |
Plate 3 YFP: 200.000 cells per well
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 500µl virus (5) | 500µl virus (9) |
| B | control, no virus | 500µl virus (9) | 500µl virus (5) |
- (1)= 10µg RC, 10µg pHelper, 10µg GOI (YFP); pH(2xHBS)=7,06
- (2)= 10µg RC, 10µg pHelper, 10µg GOI (YFP); pH(2xHBS)=7,08
- (3)= 10µg RC, 10µg pHelper, 10µg GOI (YFP); pH(2xHBS)=7,10
- (4)= 10µg RC, 10µg pHelper, 10µg GOI (YFP); pH(2xHBS)=7,12
- (5)= 10µg RC, 10µg pHelper, 10µg GOI (YFP); pH(2xHBS)=7,14
- (9)= 10µg RC, 10µg pHelper, 3,3µg GOI (eGFP)
the Results
AAV-Harvesting is at 24.8, same day Transduction and FACS will be done at 26.8
As you can see the 2xHBS with pH 4.10 performed best, in future experiments this buffer will be used.
XXXXXXXXXXXXXXXXXXXXXXXXXX
Checking transduction efficiency via fluorescence microscopy
the motivation
The transduction efficiency from the viral stocks which were created with different amounts of AAV293 was checked.
The plan
- plate 1:
- stock 1 (with 100.000 cells)
- stock 2 (with 200.000 cells)
- plate 2:
- stock 3 (with 400.000 cells)
- stock 4 (with 500.000 cells)
- plate 3:
- stock 5 (with 800.000 cells)
- stock 6 (with 1.000.000 cells)
- plate 4:
- stock 7 (with 1.200.000 cells)
- stock 8 (with 1.500.000 cells)
- plate 5:
- stock 9 (with 1.750.000 cells)
- stock 10 (with 1.750.000 cells)
The results
the optimal confluence of AAv293 cells is between 100 000 and 800 000 cells. This will be the field of investigation in the next experiments
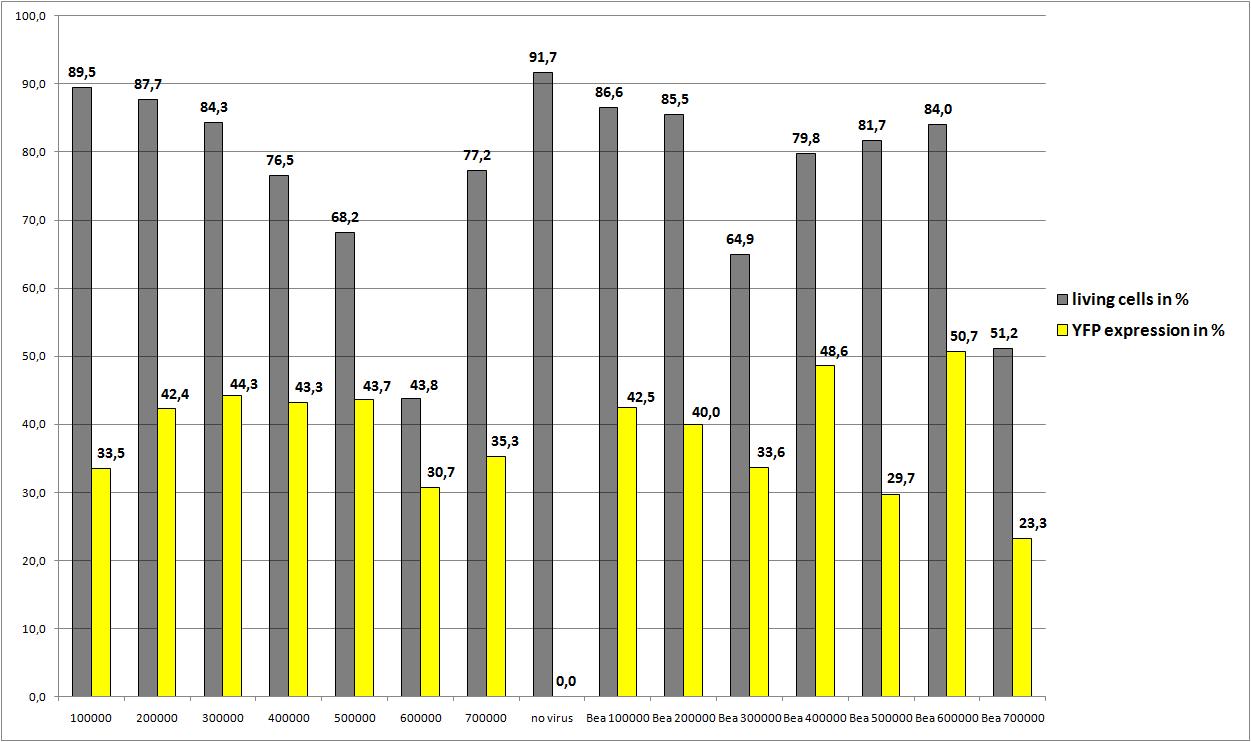
25.8: Transfection of AAV293 with different amounts of cells, and two plasmid concentrations
The motivation
We want to define the final amount of AAV293 per 10 cm2 for optimal AAV-Production
The plan
- 100.000
- 100.000
- 200.000
- 200.000
- 300.000
- 300.000
- 400.000
- 400.000
- 500.000
- 500.000
- 600.000
- 600.000
- 700.000
- 700.000
10µg RC P158a four times mutant, 10µg pHelper, 3,3 µg mVenus P162 were pipetted on plates: 2, 4, 6, 8, 10 , 12, 14
3,3µg RC P158a four times mutant, 3,3µg pHelper, 3,3 µg mVenus P262 were pipetted on plates: 1, 3, 5, 7, 9, 11, 13
| Days | 22.8 | 23.8 | 24.8 | 25.8 | 26.8 | 27.8 | 28.8 | 29.8 | 30.8 |
|---|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293 | - | Transfection | - | - | Seeding HT | Harvest + Transduction | - | FACS |
Data were not reliable (The amount of Goi (3,3 µg instead of 10µg) was not sufficient enough)so the average YFP expression is quite low, but the remarkable note is: Beas construct (pSB1C3_leftITR_CMV_betaglobin_mVenus_hGH_rightITR) fusioned from the created Biobricks still works!
30.8 Seeding AAV293 for 10xTransfection
The motivation
We want to create ten hypothetical identical viral stocks to estimate the standard deviation.
The plan
| Days | 30.8 | 31.8 | 1.9 | 2.9 | 3.9 | 4.9 | 5.9 | 6.9 |
|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293 | - | Transfection | - | Seeding HT | Harvest + Transduction | FACS |
Transfection will be done with:
- RepCap:P357 10µg =>conc.:1274,8ng/µl used amount: 7,84µl
- pHelper:P356 10µg =>conc.:1068ng/µl used amount: 9,36µl
- mVenus:P261 => excel sheet P263: 10µg =>conc.:979ng/µl used amount: 10,21 µl
0,5 ml of each stock become deepfreeze in.
One stock will be completely used for Transduction 9,5 ml (rest of the stock) * 0,5 ml (amount per transduction) => 19 values for estimating standard derivation.
XXXXXXX getting valide data of 10µg samples
The motivation
we want to check the YFP expression
The plan
| Days | 30.8 | 31.8 | 1.9 | 2.9 |
|---|---|---|---|---|
| Action | seeding HT | Transduction | - | FACS |
One stock will be completely used for Transduction 9,5 ml (rest of the stock) * 0,5 ml (amount per transduction) => 19 values for estimating standard derivation.
the results
28.8 Seeding AAV293 for checking functionality of RC vector
The motivation
We want to check different R/C constructs, additionally we check if the amount of viral.!!!!!!!!!!!!!!!!!
The plan
| Days | 28.8 | 29.8 | 30.8 | 31.9 | 1.9 | 2.9 | 3.9 | 4.9 | 5.9 | 6.9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293 | - | - | Transfection | - | - | Harvest+Seeding HT | Transduction | - | FACS |
Transfection was done with:
- RepCap:P326 => 2481 ng/µl used amount: 4µl => 10µg
- RepCap:P325 => 2051 ng/µl used amount: 4,87µl => 10µg
- pHelper:P356 => 684 ng/µl used amount: 414,62µl => 10µg
- mVenus:P262 => 1148 ng/µl used amount: 8,7µl => 10µg
The results
Transfection will be done with:
- RepCap:P357 10µg =>conc.:1274,8ng/µl used amount: 7,84µl
- pHelper:P356 10µg =>conc.:1068ng/µl used amount: 9,36µl
- mVenus:P261 => excel sheet P263: 10µg =>conc.:979ng/µl used amount: 10,21 µl
4.9 Seeding cells for checking constructs (GOIs) with/without HGH and beta globin and reassembled as control
The motivation
We want to check if these reassmbled constructs (GOIs) still work!
- P269 lITR_CMV_beta-globin_mVenus_HGH_rITR = 325 ng/µl => 9,66 µl
- P270 lITR_CMV_beta-globin_mVenus_HGH_rITR = 561 ng/µl => 7,26 µl
- P377 lITR_CMV_mVenus_HGH_rITR = 325,04 ng/µl => 30,76 µl
- P378 lITR_CMV_beta-globin_mVenus_rITR = 561 ng/µl => 17,8µl
All constructs are completed with p50 R/C =>> 26,45 µl and pHELPER 14,61µl
The plan
| Days | 4.9 | 6.9 | 7.9 | 8.9 | 9.9 | 10.9 | 11.9 | 12.9 |
|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293 | - | Transfection | - | Seeding HT | Harvest + Transduction | FACS |
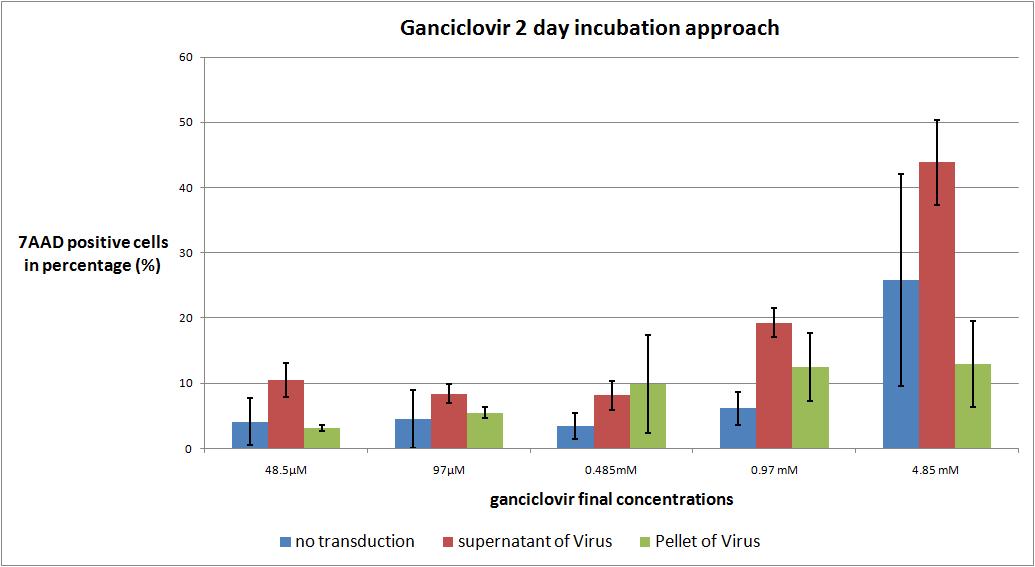
6.9 checking five ganciclovir concentrations for two day incubation approach optimal medication
The motivation
we want to check the optimal ganciclovir concentration, we use the TK/GMK Stocks (10 µg from each plasmid) from 24.8 with Buffer pH 7,10 and the pH12 stock.
The plan
| Days | 3.9 | 4.9 | 5.9 | 6.9 |
|---|---|---|---|---|
| Action | seeding HT | Transduction | - | FACS |
We want to use five different ganciclovir concentrations (the concentrations are absolute to the volume in the wells):
- 48.5µM
- 97 µM
- 0.485 mM
- 0.97 mM
- 4.85 mM
22.9 Transfection with Volkers R/C constructs
The motivation
We want to check if these R/C still work!
The plan
| Days | 20.9 | 21.9 | 22.9 | 23.9 | 24.9 | 25.9 | 26.9 | 27.9 | 28.9 |
|---|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293: 4*6wells | - | Transfection: 4 constructs | - | - | Seeding HT | Harvest + Transduction | - | FACS |
The results
On the 23.9 it was shure that something went wrong with the nomenclature, so this approach is definitely not working => threwn away!
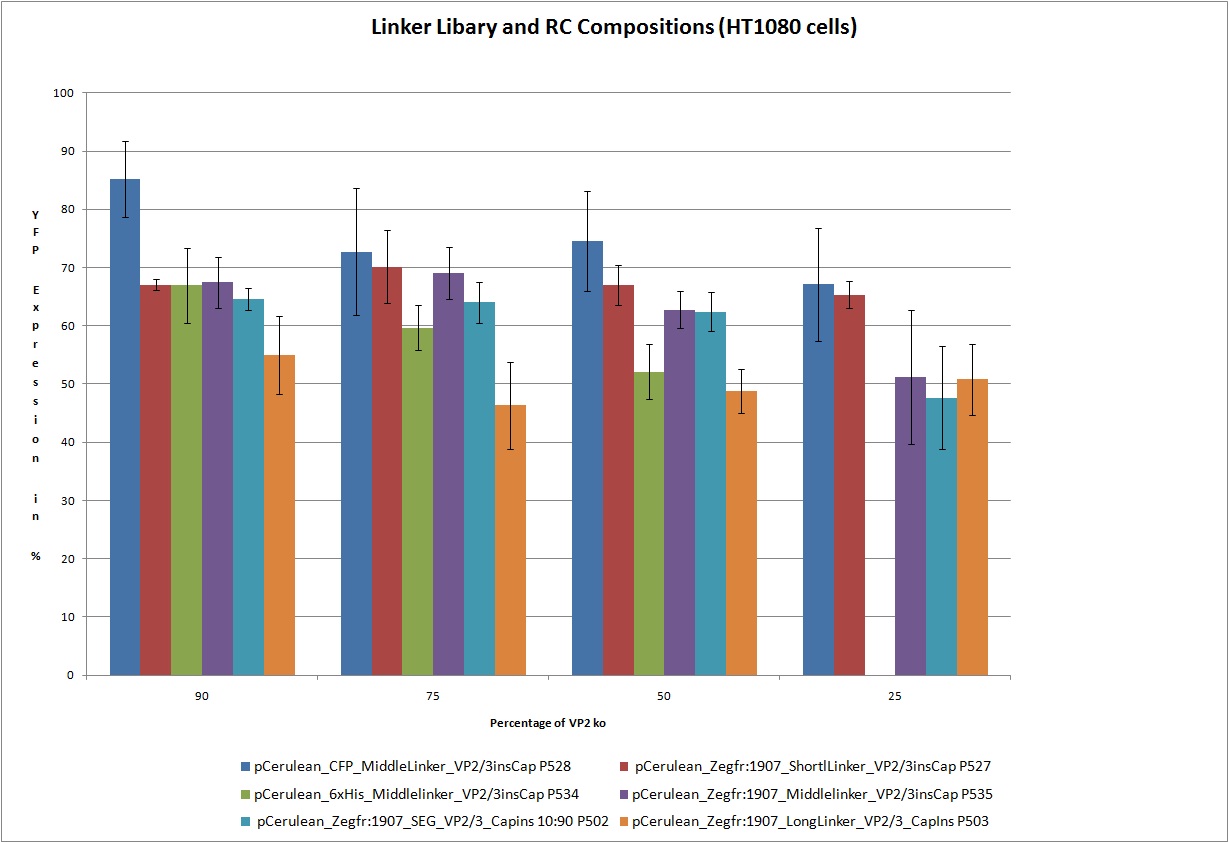
22.9 Testing of Hannas 6 VP-constructs with different compositions (10%, 25%, 50% and 75% fractions) with a VP2-knockout construct
The motivation
Test the 18 constructs for functionality on A431 and HT1080 cells.
The plan
| Days | 20.9 | 21.9 | 22.9 | 23.9 | 24.9 | 25.9 | 26.9 | 27.9 | 28.9 |
|---|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293 | - | Transfection | - | - | Seeding HT | Harvest + Transduction | - | FACS |
22.9 Seeding cells for testing pTERT_mVenus in R/C P326 and P431
The motivation
We want to check if the pTERT-promoter is realiable for tumor specific gene expression.
The plan
| Days | 22.9 | 23.9 | 24.9 | 25.9 | 26.9 | 27.9 | 28.9 | 29.9 | 30.9 | 1.10 | 2.10 | 3.10 | 4.10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293 | - | Transfection | - | - | Seeding HT | Harvest | - | - | Seeding HT, A293 and A431 | Transduction | FACS |
The results
28.9 Seeding cells for testing loop insertions
The motivation
The plan
| Days | 28.9 | 29.9 | 30.9 | 1.10 | 2.10 | 3.10 | 4.10 | 5.10 | 6.10 |
|---|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293 | - | Transfection | - | - | Seeding HT | Harvest + Transduction | - | FACS |
The results
Experiment
The motivation
The plan
| Days | 22.9 | 23.9 | 24.9 | 25.9 | 26.9 | 27.9 | 28.9 | 29.9 | 30.9 |
|---|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293 | - | Transfection | - | - | Seeding HT | Harvest + Transduction | - | FACS |
The results
Citations
[1] Media:Freiburg10_Stratagene_AAV_helper_free_manual.pdf
[2] [http://www.copewithcytokines.de/cope.cgi?key=A431 Titel einfügen]
[3] Read, A. P.; Strachan, T. (1999). "Chapter 18: Cancer Genetics". Human molecular genetics 2. New York: Wiley. ISBN 0-471-33061-2.
[4] [http://www.biomol.de/dateien/infos_nr778.gif Titel einfügen]
[5] Media:Freiburg10 Titration of AAV-2 particles via a novel capsid ELISA.pdf
 "
"