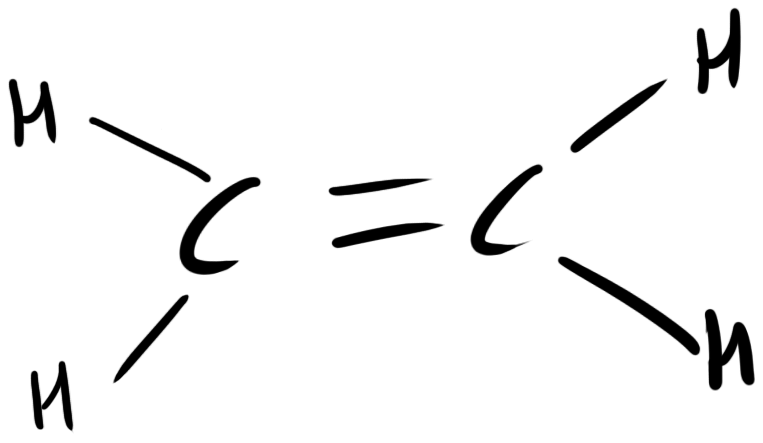Team:Monash Australia/Project
From 2010.igem.org
| (49 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | __NOTOC__ | ||
{{Template:Team:Monash_Australia/Nav2}} | {{Template:Team:Monash_Australia/Nav2}} | ||
| - | |||
| - | |||
| - | |||
| - | + | == Project overview == | |
| - | + | Monash University has a strong push for sustainability and this has rubbed off on us as students. We are passionate about using synthetic biology to reduce the impact humans have on the planet and this inspired our first iGEM project. | |
| - | + | ||
| - | + | A hot topic in Australia is the billions of non-degradable and non-renewable plastic bags that are used every year around the country, which go on to pollute waterways and take up space in landfills already packed to the limit. We decided to come up with a better way to produce poly-ethylene, the most widely used plastic compound. Not only plastic bags, but almost every plastic good imaginable from take-away food containers to rainwater tanks is manufactured from ethylene. Currently, ethylene is produced from petrochemicals and thus comes with a heavy environmental footprint. The way forward, we believe, is to take advantage of the ethylene production systems in plants and thus make ethylene manufacture both renewable and environmentally friendly. | |
| - | + | ||
| - | + | [[Image:Monash_2010_Ethylene.png|200px|thumb|left|Structure of ethylene]] | |
| - | + | ||
| - | + | <b>So what is ethylene used for?</b> | |
| - | < | + | Just about everything you do today will have you come in contact with an ethylene based product. One of the most common ethylene based products is the humble plastic shopping bag. They come in all different shapes and sizes and can be found in every country in the world. Ethylene can be polymerised to create polyethylene and is a feedstock for many other plastics, including PVC, polyester and polystyrene. However, it doesn’t stop there: ethylene is also used to produce products as varied as detergent, anti-freeze, alcohol, cosmetics, and bulletproof vests. Due to its combustible properties, it has considerable promise as a fuel for vehicles. It can even be used to ripen bananas and enhance latex production from rubber trees! |
| + | |||
| + | |||
| + | <b>How do we currently make ethylene?</b> | ||
| + | |||
| + | Currently ethylene is produced from oil or natural gas by ‘steam cracking’. This requires a huge amount of energybecause the hydrocarbons must be heated to 750–950 °C, and then immediately quenched to -157 °C to stop free radical reactions. Ethylene is separated from the resulting complex mixture by repeated compression and distillation. | ||
| + | The average ethylene production plant has a 34,000 kW cracked gas compressor, a 22,000 kW propylene compressor, and a 11,000 kW ethylene compressor, which combine to one big power bill and an enormous carbon footprint! The fossil fuels used are also non-renewable resources that may soon be in short supply. Oil, particularly, is a major environmental hazard, as we saw recently with the unprecedented spill in the Gulf of Mexico. | ||
| + | |||
| + | |||
| + | <b>How do plants make ethylene?</b> | ||
| + | |||
| + | Plants synthesise ethylene naturally. Ethylene is a plant hormone, which can induce plants to grow and fruit to ripen. Ethylene biosynthesis occurs in three steps, starting with the conversion of the amino acid methionine to S-adenosyl-L-methionine (SAM) by the enzyme SAM synthase. SAM is then converted to 1-aminocyclopropane-1-carboxylic-acid (ACC) by the enzyme ACC synthase. The final step involves the action of the enzyme ACC-oxidase to oxidise ACC to ethylene. | ||
| + | |||
| + | ==So what are the more common items we can relate to?== | ||
<Center> | <Center> | ||
| - | + | <gallery> | |
| - | + | Image:Monash_Australia_LLDPE.png|Linear Low Density Polyethylene - Plastic wrap | |
| - | + | Image:Monash_Australia_LDPE.png|Low Density Polyethylene - Soft Plastic Bottles | |
| - | + | Image:Monash_Australia_MDPE.png|Medium Density Polyethylene - Pipes | |
| - | + | Image:Monash_Australia_HDPE.png|High Density Polyethylene - Hard Plastic bottles | |
| - | + | Image:Monash_Australia_UHMWPE.png|Ultra High molecular weight Polyethylene - Bullet proof vests | |
| - | + | Image:Monash_Australia_pvc.png|PVC (polyvinal chloride)- Pipes | |
| - | + | Image:Monash_Australia_polystryene.png|Polystryene - Packing material and insulation | |
| - | + | Image:Monash_Australia_antifreeze.png|Ethylene Glycol - Anti-Freeze | |
| - | + | </gallery> | |
| - | + | </center> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | |||
| - | |||
| - | |||
| - | + | ==A case study: plastic water bottles== | |
| - | + | ||
| - | + | ||
| - | + | [[Image:Monash_Australia_bottledwater.jpg|200px|thumb|left|Polyethylene terephthalate (PET)]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | Let’s explore example of an ethylene based product: disposable plastic water bottles. These bottles are made from PolyEthylene Terephthalate (PET), and according to the Pacific Institute and the Bottled Water Alliance, over 15,000 tonnes of PET was used in packaging for bottled water in 2009-10 in Australia, The manufacture of one tonne of PET produces about three tonnes of Carbon Dioxide, equating to over 45,000 tonnes of CO2 released into the atmosphere per year due to these plastic bottles. To manufacture the PET, approximately 53 million litres of oil was used in 2009-10 in the production of bottles for water in Australia. These figures exclude impacts from the mining and transportation of crude materials. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | On their own, those numbers are incredible, but when you take into account every other bottled product - soft drinks, fruit juice, sport drinks, milk, etc – it is clear that the manufacture of plastic bottles consumes an absolutely immense amount of energy and results in a tremendous amount of carbon pollution. Given modern society’s love affair with plastic bottles, policy-based mechanisms to discourage their use are unlikely to be effective. What could work is a technological solution, by which ethylene manufacture is decoupled from fossil fuels. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | Our vision is to genetically engineer <i>Escherichia coli</i> to produce ethylene at room temperature, at low cost, with low energy reqirements and using renewable organic feedstocks. | |
| - | + | ||
| - | + | (Sources: [http://www.pacinst.org/topics/water_and_sustainability/bottled_water/bottled_water_and_energy.html The Pacific Institute] and [http://www.bottledwateralliance.com/ The bottled Water Alliance]) | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
== Experimental plan == | == Experimental plan == | ||
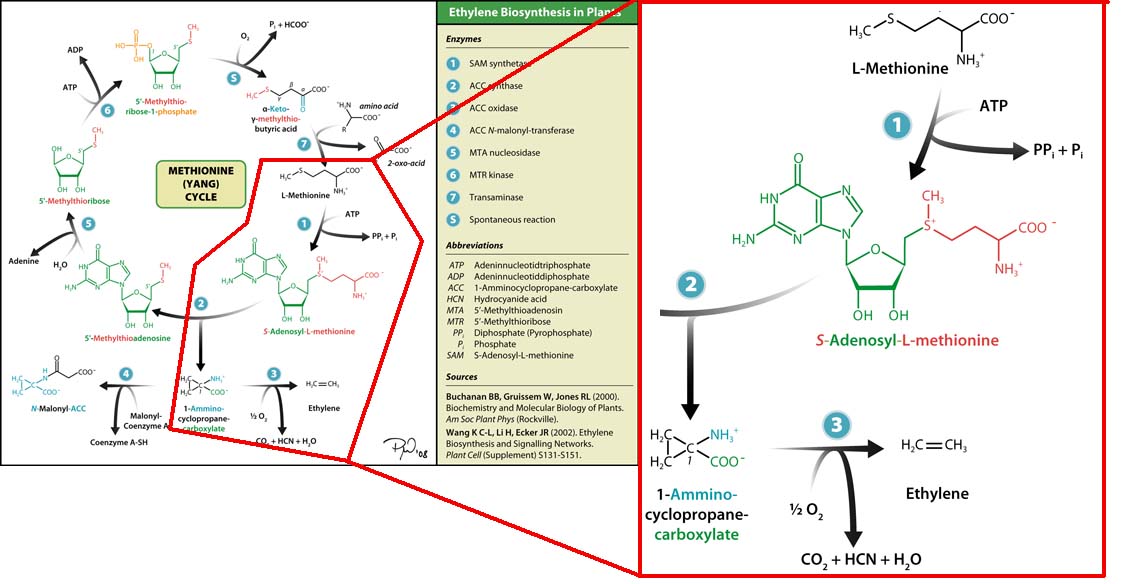
| - | + | [[Image:Monash_Australia_Yang-cycle.png|200px|thumb|left|Yang cycle]] | |
| + | |||
| + | To make this vision a reality, we attempted to transfer the plant ethylene biosynthesis pathway into <i>E. coli</i>. Plants produce ethylene through the Yang Cycle, which uses methionine as a base molecule to produce several different products. We studied the enzymes involved and designed a genetic circuit composed of SAM synthase, ACC synthase and ACC oxidase. E coli already possesses a gene for SAM synthase, so we added a second copy to our construct to ramp up production. | ||
| + | |||
| + | ACC synthase and ACC oxidase are only found in plants, so we explored enzyme characterisation databases to find the most efficient and specific catalysts from the plant world. Our analysis pointed to apple for ACC synthase and tomato for ACC oxidase. With this in silico work done, our plan was to have all three genes synthesised by Mr. Gene after codon optimising them for <i>E. coli</i>. | ||
| - | + | We would then add ribosome binding sites and link these three genes together in one construct for efficient ethylene production from <i>E. coli</i>. Naturally, all of the constructs would be made available as biobricks for future iGEMers. Apart from industrial-scale ethylene production, these biobricks could potentially be used for future projects involving cellular signalling through ethylene receptors derived from plants. | |
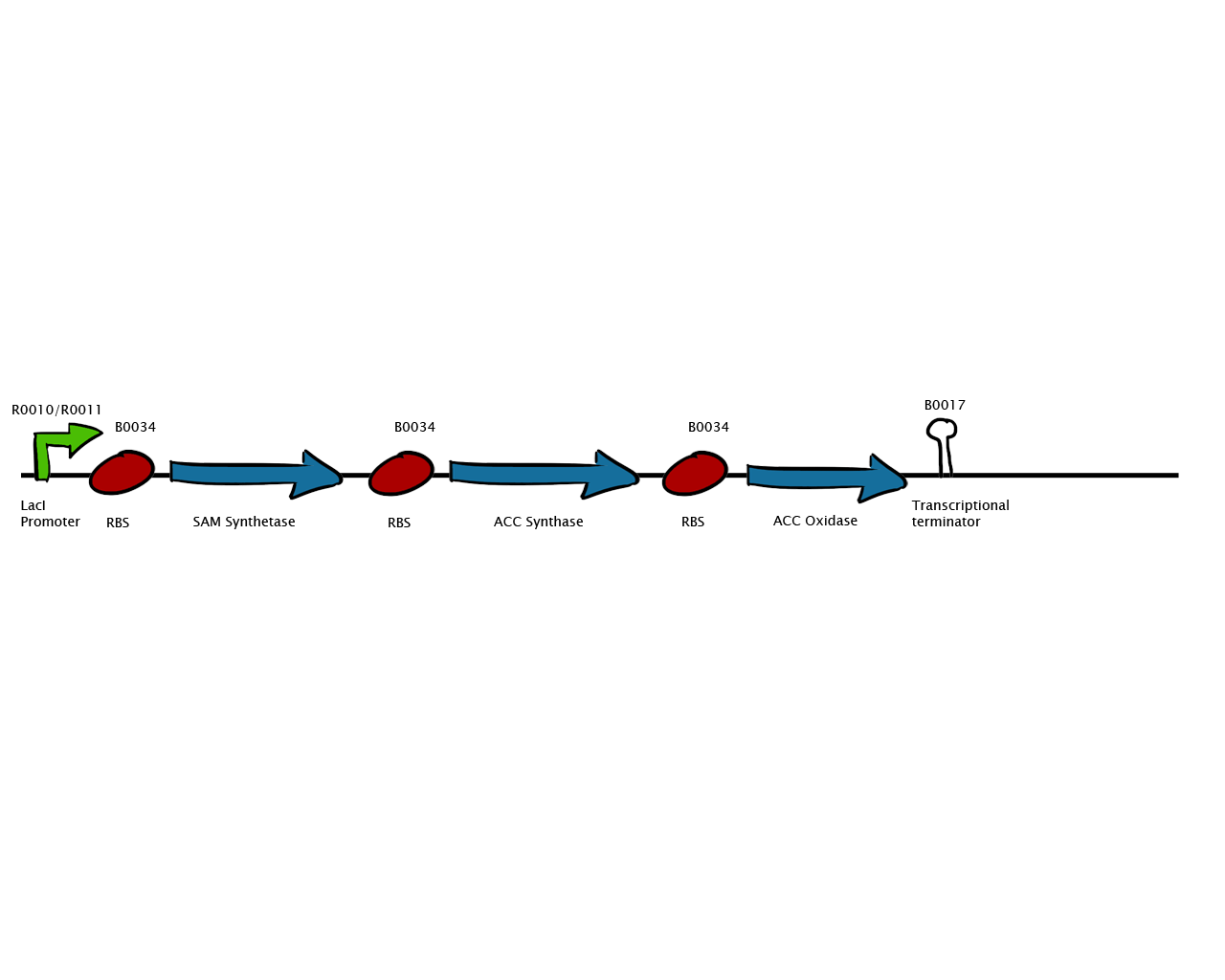
| - | + | [[Image:Monash_2010_construct.png|200px|thumb|left|Construct design]] | |
Latest revision as of 10:22, 27 October 2010

|
|
|||
Project overview
Monash University has a strong push for sustainability and this has rubbed off on us as students. We are passionate about using synthetic biology to reduce the impact humans have on the planet and this inspired our first iGEM project.
A hot topic in Australia is the billions of non-degradable and non-renewable plastic bags that are used every year around the country, which go on to pollute waterways and take up space in landfills already packed to the limit. We decided to come up with a better way to produce poly-ethylene, the most widely used plastic compound. Not only plastic bags, but almost every plastic good imaginable from take-away food containers to rainwater tanks is manufactured from ethylene. Currently, ethylene is produced from petrochemicals and thus comes with a heavy environmental footprint. The way forward, we believe, is to take advantage of the ethylene production systems in plants and thus make ethylene manufacture both renewable and environmentally friendly.
So what is ethylene used for?
Just about everything you do today will have you come in contact with an ethylene based product. One of the most common ethylene based products is the humble plastic shopping bag. They come in all different shapes and sizes and can be found in every country in the world. Ethylene can be polymerised to create polyethylene and is a feedstock for many other plastics, including PVC, polyester and polystyrene. However, it doesn’t stop there: ethylene is also used to produce products as varied as detergent, anti-freeze, alcohol, cosmetics, and bulletproof vests. Due to its combustible properties, it has considerable promise as a fuel for vehicles. It can even be used to ripen bananas and enhance latex production from rubber trees!
How do we currently make ethylene?
Currently ethylene is produced from oil or natural gas by ‘steam cracking’. This requires a huge amount of energybecause the hydrocarbons must be heated to 750–950 °C, and then immediately quenched to -157 °C to stop free radical reactions. Ethylene is separated from the resulting complex mixture by repeated compression and distillation. The average ethylene production plant has a 34,000 kW cracked gas compressor, a 22,000 kW propylene compressor, and a 11,000 kW ethylene compressor, which combine to one big power bill and an enormous carbon footprint! The fossil fuels used are also non-renewable resources that may soon be in short supply. Oil, particularly, is a major environmental hazard, as we saw recently with the unprecedented spill in the Gulf of Mexico.
How do plants make ethylene?
Plants synthesise ethylene naturally. Ethylene is a plant hormone, which can induce plants to grow and fruit to ripen. Ethylene biosynthesis occurs in three steps, starting with the conversion of the amino acid methionine to S-adenosyl-L-methionine (SAM) by the enzyme SAM synthase. SAM is then converted to 1-aminocyclopropane-1-carboxylic-acid (ACC) by the enzyme ACC synthase. The final step involves the action of the enzyme ACC-oxidase to oxidise ACC to ethylene.
So what are the more common items we can relate to?
A case study: plastic water bottles
Let’s explore example of an ethylene based product: disposable plastic water bottles. These bottles are made from PolyEthylene Terephthalate (PET), and according to the Pacific Institute and the Bottled Water Alliance, over 15,000 tonnes of PET was used in packaging for bottled water in 2009-10 in Australia, The manufacture of one tonne of PET produces about three tonnes of Carbon Dioxide, equating to over 45,000 tonnes of CO2 released into the atmosphere per year due to these plastic bottles. To manufacture the PET, approximately 53 million litres of oil was used in 2009-10 in the production of bottles for water in Australia. These figures exclude impacts from the mining and transportation of crude materials.
On their own, those numbers are incredible, but when you take into account every other bottled product - soft drinks, fruit juice, sport drinks, milk, etc – it is clear that the manufacture of plastic bottles consumes an absolutely immense amount of energy and results in a tremendous amount of carbon pollution. Given modern society’s love affair with plastic bottles, policy-based mechanisms to discourage their use are unlikely to be effective. What could work is a technological solution, by which ethylene manufacture is decoupled from fossil fuels.
Our vision is to genetically engineer Escherichia coli to produce ethylene at room temperature, at low cost, with low energy reqirements and using renewable organic feedstocks.
(Sources: [http://www.pacinst.org/topics/water_and_sustainability/bottled_water/bottled_water_and_energy.html The Pacific Institute] and [http://www.bottledwateralliance.com/ The bottled Water Alliance])
Experimental plan
To make this vision a reality, we attempted to transfer the plant ethylene biosynthesis pathway into E. coli. Plants produce ethylene through the Yang Cycle, which uses methionine as a base molecule to produce several different products. We studied the enzymes involved and designed a genetic circuit composed of SAM synthase, ACC synthase and ACC oxidase. E coli already possesses a gene for SAM synthase, so we added a second copy to our construct to ramp up production.
ACC synthase and ACC oxidase are only found in plants, so we explored enzyme characterisation databases to find the most efficient and specific catalysts from the plant world. Our analysis pointed to apple for ACC synthase and tomato for ACC oxidase. With this in silico work done, our plan was to have all three genes synthesised by Mr. Gene after codon optimising them for E. coli.
We would then add ribosome binding sites and link these three genes together in one construct for efficient ethylene production from E. coli. Naturally, all of the constructs would be made available as biobricks for future iGEMers. Apart from industrial-scale ethylene production, these biobricks could potentially be used for future projects involving cellular signalling through ethylene receptors derived from plants.
 "
"