Team:TU Delft/9 August 2010 content
From 2010.igem.org
Contents |
Lab work
Alkane degradation
Colony PCR
Friday, a colony PCR on colonies from a plate with transformants with BioBrick 013AK did not give positive results. But this doesn't mean that none of the colonies are correct. To check we did another colony PCR today on 8 more colonies of the AMP plate as well as the KAN plate. Hopefully a the PCR product of a colony will show the correct length.
Lane description
| # | Description | Primers | Expected length (bp) | ✓ | ✗ |
| L | SmartLadder | n/a | n/a | n/a | n/a |
| 1-8 | 013A | G00100 + G00101 | 1929 | none | all |
| 9-16 | 013K | G00100 + G00101 | 1929 | none | all |
| L | SmartLadder | n/a | n/a | n/a | n/a |
Unfortunately none of the PCR products seemed to have the correct length. We'll try to make the BioBrick again.
Digestion, Ligation, Transformation
A digestion was done on a number of BioBricks for the production of new BioBricks.
| # | Sample | Enzyme 1 | Enzyme 2 | Enzyme 3 | Buffer | BSA | Needed fragment |
| 1 | 009A | EcoRI | SpeI | PvuI | 2 (Biolabs) | ✓ | ‘E - J61100 - rubA4 - S’ |
| 2 | 012K | EcoRI | XbaI | ✗ | 2 (Biolabs) | ✓ | ‘E – J61100 - rubR - B0015 – X’ |
| 3 | 017A | SpeI | PstI | 2 (Biolabs) | ✓ | ‘P - pSB1A2 – J61100 - ladA – S’ | |
| 4 | 018A | XbaI | PstI | PvuI | 3 (Biolabs) | ✓ | ‘X – J61107 - ALDH – P’ |
The digestion was checked on a gel, together with the uncut BioBricks:
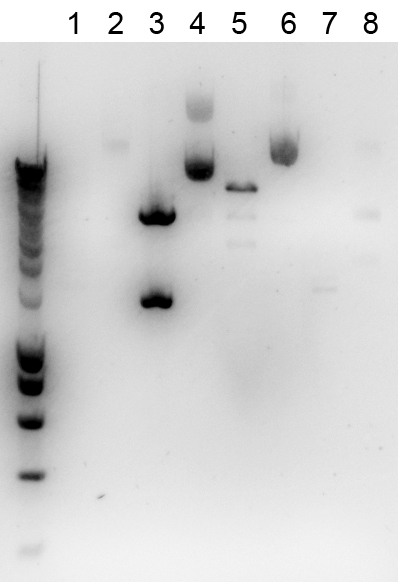
Lane description
| # | Description | Expected size (bp) | OK? | # | Description | Expected size (bp) | OK? |
| L | Smartladder (3μl) | n/a | n/a | 5 | 017A cut | ||
| 1 | 009A cut | 6 | 017A uncut | n/a | |||
| 2 | 009A uncut | n/a | 7 | 018A cut | |||
| 3 | 012K cut | 8 | 018A uncut | n/a | |||
| 4 | 012K uncut | n/a |
For some samples a too low concentration was loaded, but furthermore the digestions looked ok.
Ligation
Following the digestions, the fragments were [Team:TU_Delft/protocols/ligation ligated]] for 4 hours.
| # | BioBrick | Fragment 1 | Recipient vector |
| 1 | 013AK | 10 μL ‘E – J61100 - rubA4 – S’ | 10 μL ‘X – J61100 - rubR - B0015 - pSB1AK3 – E’ |
| 2 | 020A | 10 μL ‘P - pSB1A2 – J61100 - ladA – S’ | 10 μL ‘X – J61107 - ALDH – P’ |
Emulsifier
Today we tried to see whether P. Putida excreeds an emulsifier. There were 3 samples:
- P. Putida grown over night on Glucose (OD: 1.559)
- P. Putida grown over night on Octane (OD: 0.838)
- M9 medium (negative control) (OD: 0.000)
Therefore we used the following protocol:
Materials:
- 25 mM triethanolamine (TEA) buffer, pH 8
- 1% lysozyme in TEA
- 4M urea
- 10% streptomycin in TEA
- Glass beads
- 25 mM Tris buffer (pH 8)
Method:
- Harvest 25 mL bacterial cells by centrifugation at 10.000 rpm for 10 min
- Collect the cell free supernatant and store it
- Wash pellet with TEA buffer
- Freeze pellet in -70 C for 15 min
- Resuspend pellet 2 mL TEA + 40 ul 1% lysozyme (final concentration 0.02%)
- Incubate for 10 min at RT
- Disrupt cells with glass beads. Vortex for 10 minutes
- Centrifuge at 14.000 rpm for 30 min at 4 C (protein is in supernatant)
- Aliquot 1.8 mL supernatant into fresh eppendorf tubes and add 200 ul 10% streptomycin in TEA (final concentration 1%)
- Incubate 10 min at RT
- Centrifuge at 14.000 rpm for 30 min at 4 C (protein is in pellet)
- Resuspend pellet in 4 M urea
- Centrifuge at 14.000 rpm for 30 min at 4 C (protein is in supernatant)
The samples were tested according to the emulsification assay protocol (@TODO: link):
| # | Buffer (ul) | Hexane (ul) | Sample (ul) | Absorbance (660nm) |
| 1 | 950 | 50 | 0.272 | |
| 2 | 900 | 50 | 50 M9 | 0.141 |
| 3 | 950 | 0 | 50 M9 | 0.001 |
| 4 | 900 | 50 | 50 Glucose culture (sup) | 0.100 |
| 5 | 950 | 0 | 50 Glucose culture (sup) | 0.023 |
| 6 | 900 | 50 | 50 Octane culture (sup) | 0.161 |
| 7 | 950 | 0 | 50 Octane culture (sup) | 0.013 |
| 8 | 900 | 50 | 50 Glucose culture (pellet) | 0.118 |
| 9 | 950 | 0 | 50 Glucose culture (pellet) | 0.014 |
| 10 | 900 | 50 | 50 Octane culture (pellet) | 0.168 |
| 11 | 950 | 0 | 50 Octane culture (pellet) | - 0.002 |
| 12 | 900 | 50 | 50 4 M Urea | 0.175 |
| 13 | 950 | 0 | 50 4 M Urea | - 0.001 |
The conclusion is that with the additional of M9 or 4 M Urea the amount of hexane in the Tris buffer decreases. When the samples are used the emulsification becomes even lower. Probably due to the addition of the proteins the total amount of hydrophobic interfaces already increases. Therefore, a lower amount of hexane can be dissolved.
 "
"
