Team:Stockholm/18 August 2010
From 2010.igem.org
Contents |
Andreas
Site-directed mutagenesis of SOD & yCCS
Continued from 17/8
Plasmid prep
From 17/8 ON cultures
Elution volume: 70 μl dH2O
| DNA concentrations | ||
|---|---|---|
| Sample | Conc [ng/μl] | A260/A280 |
| SC1→pSB1C3.m-SOD 1 | 107.9 | 1.84 |
| SC2→pSB1C3.m-SOD 2 | 105.5 | 1.83 |
| yC1→pSB1C3.m-yCCS 1 | 101.2 | 1.86 |
| yC4→pSB1C3.m-yCCS 4 | 106.4 | 1.80 |
Nina
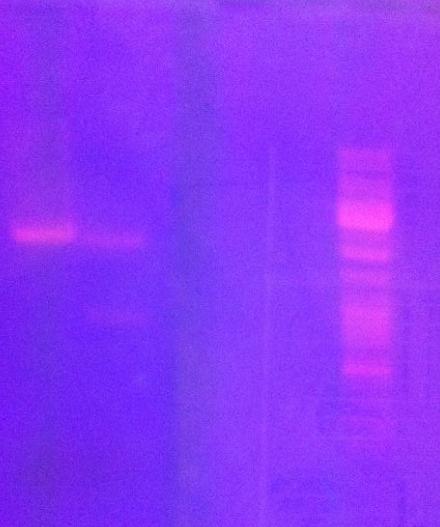
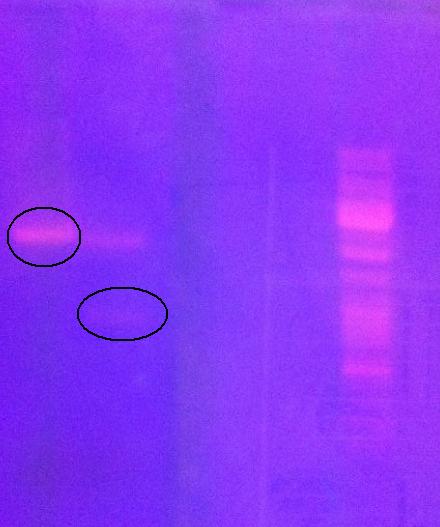
Digest protein A and IgG protease on gel
I ran previously digested protein A and IgG protease in an agarose gel 1 % (110 V) in order to do a gel clean up. This was because I did not obtain good results from colony PCR of previously ligated protein A with IgG protease (fusionprotein). Therefore, I redo the ligation and transformation into e.coli, however I this time include a gel clean up of the digested samples in order to exclude interfering material in the tube when ligating.
In addition I found out from the sequencing that the protein A I have in the bank vector does not consist of two Z domains (ZZ domain), instead I only have one of the domains (Z domain, 180 nt).
Arrangement on gel:
Ladder: GeneRuler™ DNA Ladder Mix, ready-to-use, 100-10,000 bp Fermentas
Gel clean up of protein A and IgG protease
After running the gel I cut out with a scalpel the bands that looked like they corresponded to the vector containing protein A and the IgG protease cut out of the vector.
I followed the gel clean up according to the method described in protocols.
In step 2:
- protein A = 200 mg * 3 = 600 ul of QX1
- IgG protease = 200 mg * 3 = 600 ul of QX1
In step 3:
Both of the samples were added 30 ul of QIAEX II
In step 9:
Both of the samples were incubated in RT for 5 min
Spectophotometer:
Ligation of protein A and IgG protease
The vector should be about 25 ng and the gene that I want to insert in the vector should be about 3-5 times more.
- Vector: 40 ng/ul
0.5 ul = 20 ng
- Gene: 34 ng/ul
2 ul = 68 ng
68 / 20 = 3 (times more gene than vector)
Ligation:
- Protein A (vector) 0.5 ul
- IgG protease (gene) 2 ul
- Quick ligase 1 ul
- Quicke ligase buffer 2X 3.5 ul
I incubated in RT for 15 min.
Transformation of protein A and IgG protease
I transformed the ligated product into 100 ul Top 10 cells.
I followed the procedure decribed in protocols. However, in step 1 I thawed the cells for 15 min instead of 10 min and in step 2 I added 3 ul DNA sample to the cells.
Sequencing tyrosinase
I sent two samples of the site-directed mutagenesis tyrosinase for sequencing. Colony nr 4 and 6.
Since I have the gene in the original vector I did not have any verification primers for the vector, however the site i have to control by the sequencing is in the middle of the gene and not in the beginning nor the end of the gene (which usually result in poor sequening data). Therefore, I did a dilution of the stock sample of the gene tyrosinase forward primer to suit for the standard of the seqencing.
Tyrosinase forward primer (stock): 100 uM
I wanted to have a concentration of 10 uM to send in with the sample.
100uM / X = 10uM
X = 10
Dilution must be 1:10
I prepared the 10 uM forward primer by mixing 1 ul stock sample with 9 ul H2O.
The tube to send in I prepared with:
- Vector with tyrosinase 15 ul
- Primer 10 uM 1.5 ul
Sample identification:
- Colony #4: ASB0045 330
- Colony #6: ASB0045 331
Mimmi
MITF
Gel
- No product
 |

|
 |

|
 |

|

|

|
 "
"