Team:Bielefeld-Germany/Project/Approach
From 2010.igem.org
(→Directed mutagenesis) |
|||
| Line 67: | Line 67: | ||
=== Survival of the fittest === | === Survival of the fittest === | ||
| - | We used high amounts of the antibiotic kanamycin in order to select the most specific system for our substances. By varying the amount of kanamycin we will be able to carefully select the best mutant out of our error-prone PCR tests. The mutated ''virA'' system will be induced after the error prone PCR by a mix of possible targets for the system (like | + | We used high amounts of the antibiotic kanamycin in order to select the most specific system for our substances. By varying the amount of kanamycin we will be able to carefully select the best mutant out of our error-prone PCR tests. The mutated ''virA'' system will be induced after the error prone PCR by a mix of possible targets for the system (like capsaicin, dopamin, homovanillic acid etc.). It is important to avoid any acetosyringone, so we will be able to search for systems with new targets. |
=== Directed mutagenesis === | === Directed mutagenesis === | ||
| - | Alternatively to the mutagenesis strategy we developed two | + | Alternatively to the mutagenesis strategy we developed two strategies for a directed mutagenesis: |
'''VirA_mut1''' | '''VirA_mut1''' | ||
Revision as of 02:22, 28 October 2010
Contents |
The Approach
First we looked for a sensor system which is able to detect substances and we searched for substances of interest. The system of choice had to be taken out of a different bacteria species than Escherichia coli in order to avoid any background. Therefore we checked the literature for a well reviewed sensor system, which is not part of the E. coli genome. Finally we decided to choose the phenolic sensing VirA/G signaling system of Agrobacterium tumefaciens. It naturally detects acetosyringone, which is a secondary metabolite of plants that affects bacteria as an attractant. After repeated research to check for any other possible substances which could be detected by the system, we got a really long list of possibilities and picked 'capsaicin', which is responsible for the spiciness of edibles.
The biological steps for creating a new VirA/G sensor system are:
- extract the complete VirA/G signaling system out of A. tumefaciens
- create new BioBricks out of the existing environmental parts
- get the new BioBricks working into E. coli
- choose a reporter gene as readout for the sensor system
- modify the system for sensibility and specificity by error prone PCR, find and select the most promising mutants (directed mutagenesis)
Preparing the system
We tried to work with the already existing VirA receptor BioBrick from the registry (<partinfo>K238008</partinfo>). Unfortunately this VirA BioBrick did not work. The sequence data of the BioBrick did not fit to the published VirA sequence. So we had to extract the virA gene via PCR out of the A. tumefaciens TI-plasmid by ourselves in order to create a new BioBrick.
VirA does not work without the help of the VirG protein. The existing virG gene in the iGEM registry contains illegal restriction sites. Moreover it needs the help of the RpoA Protein from A. tumefaciens to work as a transcription facor in E. coli. We changed the sequence manually and had the gene synthesized by Mr. Gene.
Starting point for BioBricks -> Go cloning
After discussing the possibility of creating only one big plasmid for mutated virA screenings, we unified that one big plasmid would minimize transformation efficiency and would be difficult to modify via error prone PCR. So we had to change the origin of a pSB1C3 plasmid in order to avoid incompatibility. Thus we cloned the R6K origin (<partinfo>J61001</partinfo>) into the <partinfo>pSB1C3</partinfo> plasmid and subsequently removed the original pMB1/ColE1 origin of replication. Therefore we digested the pSB1C3::R6K plasmid with Hin6I and seperated the resulting 6 fragments (1758 bp, 270 bp, 174 bp, 109 bp, 100 bp, 67 bp) by agarose gel electrophoresis. We extracted the largest fragment (1758 bp) and religated it. The resulting plasmid still contains 32 remaining basepairs of the pMB1 origin, but this fragment does not enable replication in pir- strains.
The error prone PCR and screening
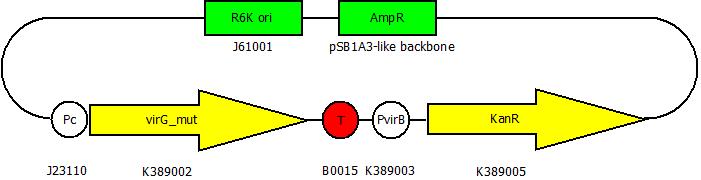
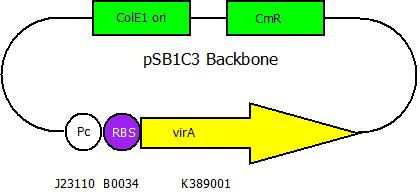
After building a plasmid with R6K ori we were able to create the two constructs. The first one will be found inside the competent bacteria cells and contains the virG gene under the constitutive promoter <partinfo>J23110</partinfo>, a terminator (<partinfo>B0017</partinfo>), a vir promoter and a readout or selection gene (luciferase, mRFP and kanamycin resistance, respectively):
The second plasmid contains the (mutated) virA gene under the control of the constitutive promoter <partinfo>J23110</partinfo> and will be transformed and modified in one step via error prone PCR.
The error prone PCR is a PCR under malfunction conditions for the polymerase. It is possible to regulate the frequency of the mutagenesis by editing unbalanced concentrations of nucleotides and co-factors to the PCR. So we are going to create a new BioBrick and transform it into our target in one step. Before we are able to do the error prone PCR we need to get the backbones done.
Survival of the fittest
We used high amounts of the antibiotic kanamycin in order to select the most specific system for our substances. By varying the amount of kanamycin we will be able to carefully select the best mutant out of our error-prone PCR tests. The mutated virA system will be induced after the error prone PCR by a mix of possible targets for the system (like capsaicin, dopamin, homovanillic acid etc.). It is important to avoid any acetosyringone, so we will be able to search for systems with new targets.
Directed mutagenesis
Alternatively to the mutagenesis strategy we developed two strategies for a directed mutagenesis:
VirA_mut1
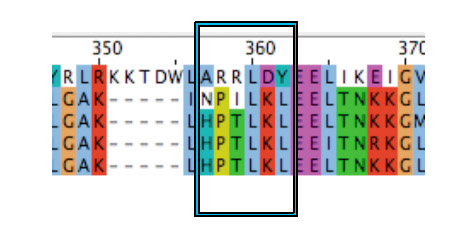
Regarding to the results from Muscle, we used site directed mutagenesis (for Protocols see: ([http://openwetware.org/wiki/Site-directed_mutagenesis OpenWet Ware])) to change VirA protein sequence leucin 293 to tyrosine (L293Y = mut1).
VirA_mut2
After VirA_mut1 with its single mutation failed working in our screening system, we decided to mutate some further amino acids in the area of the reported binding region from the animal TRPV1 receptors.
According to the literature amino acid residues from 288 to 293, regulate phenol structural specificity in the TRPV1 receptor. Substitutions in this region narrow the active phenols to those missing the para-acyl substituent ([http://www.springerlink.com/content/978-0-387-78884-5#section=169995&page=1 Lin et al., 2008]). Our VirA_mut2 construct contains the following substitutions:
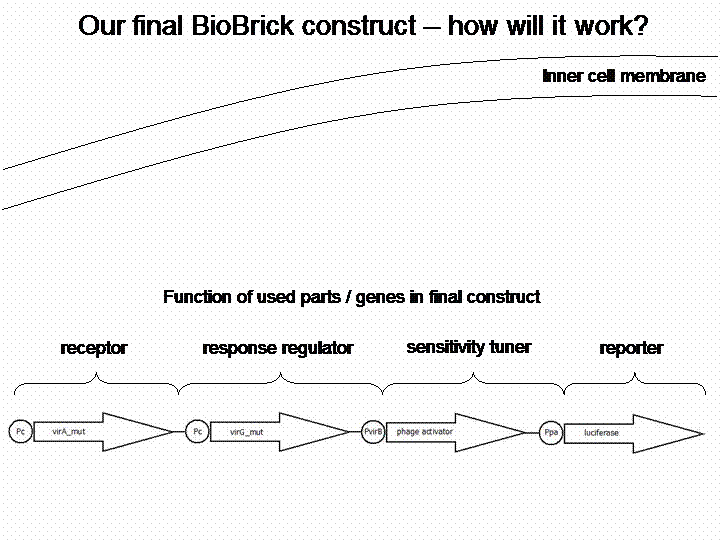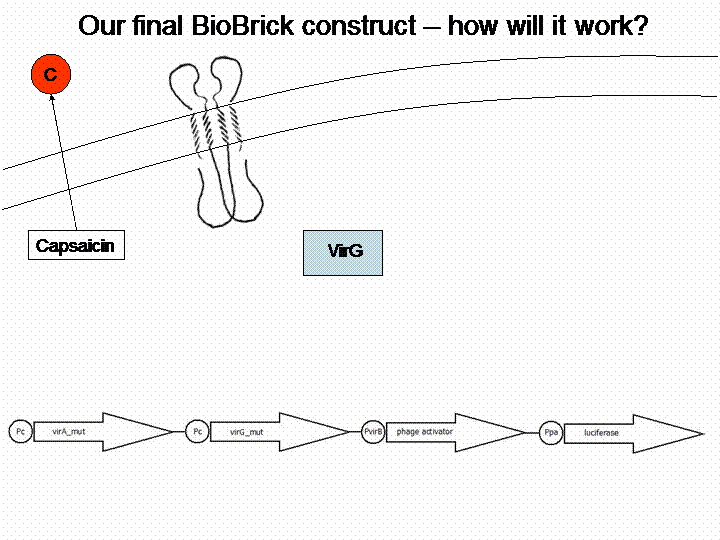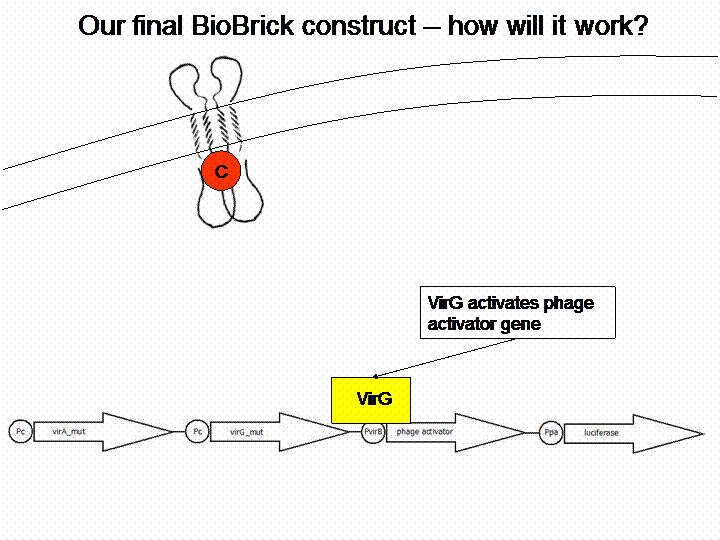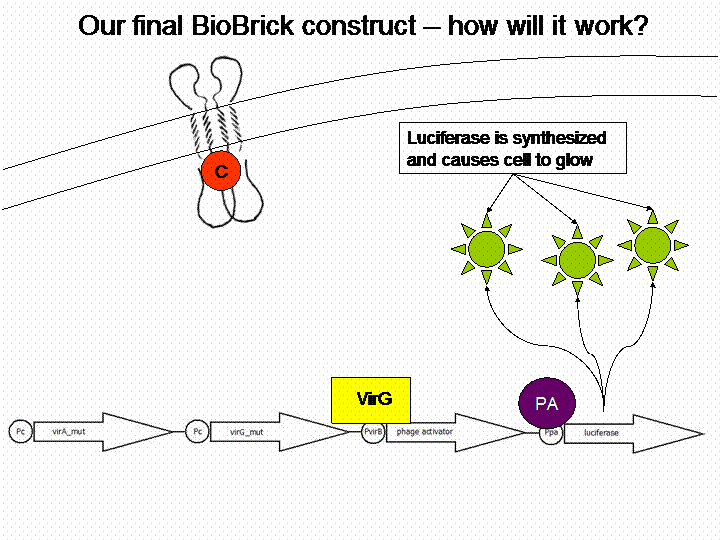
The final construct
In order to get a visible light signal by the reporter gene luciferase we integrated genetic amplifiers into the final construct to enhance this signal.
For a demonstration of this final system see the animation below:
 "
"