Team:LMU-Munich/Notebook/Apoptosis
From 2010.igem.org
(→8-24-2010) |
(→8-24-2010) |
||
| Line 722: | Line 722: | ||
* 4.5 ng (from PCR2a) 0.9µl (1:10 diluted) | * 4.5 ng (from PCR2a) 0.9µl (1:10 diluted) | ||
| - | + | ::{| | |
| - | + | |- | |
| - | + | |PCR2a | |
| - | + | |0.9 µl | |
| - | + | |- | |
| - | + | |PCR2b | |
| - | + | |0.6 µl | |
| - | + | |- | |
| - | + | |primer3 | |
| - | + | |2.5 µl | |
| - | + | |- | |
| - | + | |primer6 | |
| - | + | |2.5 µl | |
| - | + | |- | |
| - | + | |dNTPs | |
| - | + | |1 µl | |
| + | |- | ||
| + | |Pfu | ||
| + | |0.5 µl | ||
| + | |- | ||
| + | |10xbuffer | ||
| + | |5 µl | ||
| + | |- | ||
| + | |H<sub>2</sub>O | ||
| + | |37 µl | ||
| + | |} | ||
Revision as of 08:53, 25 August 2010
Some test text in bold
We created following tests:
- test4 Example of a table
this too is a table:
table with 3 cells
text
text
test text Knallroter Text test grüner text
Transforming competent cells
- eGFP Biobrick: BBa_I714891 SDY_eGFP (Kanamycin)
- TEV recogn N Degron SF3 = pDS7 (Ampicillin)
- TEV p14 recogn = 190-6 (Ampicillin)
-> Protocol: (3 Transformation)
- We added 2 µl DNA
- We plated out 200 µl
- CMV-Promoter Biobrick: BBa_J52034
-> Protocol:(4 Plasmid extraction from cells)
- Prepared overnight culture, measured concentration of DNA
-> Poor results -> thrown away
New Plasmid Extraction
- CMV-Promoter Biobrick: BBa_J52034
-> Protocol: (4 Plasmid extraction from cells)
- Plasmid concentration: 143ng/µl
- 3 ml LB-Media + 4 µl Kanamycin
- Inoculated iangeimpft) with 1 colony of BBa_I714891 -> 37°C
- for 190-6 and pDS7: 10µl Ampicillin + 10 ml LB-Media + colony of plate
- for eGFP: 13,3 µl Kanamycin + 10 ml LB-Media + 1 colony of plate
-> mixed
- plus: EcoRI (10µg/µl): 0,5 µl resp. PstI (10µg/µl): 0,5 µl
- incubated at room temperature from 12:10 to 15:00, 1 hour at 37°C, 2 hours at 60°C
- frozen at -20°C
- 1 ml of "old" culture + 3 ml LB-Media + 4 µl Kanamycin -> 37°C
Plasmid Extraction of pDS7, eGFP, 190-6
-> Protocol: (4 Plasmid extraktion from cells)
- pDS7 (458ng/µl), eGFP (55ng/µl), 190-6 (193ng/µl)
Restriction digest of pDS7, eGFP, 190-6
- with EcoRI and PstI in buffer H (for testing DNA is correct)
-> Protocol: (5 Restriction digest)
- 10µg DNA: pDS7 (2µl), eGFP (15µl), 190-6 (10µl)
Plate colonies for plasmid extrction
- CMV (Kanamycin), eGFP (Kanamycin), pDS7 (Ampicillin), 190-6 (Ampicillin))
- PhiC31o plated on Ampicillin-Agar, stored at 37°C
50% Glycerol made
- for PhiC31o glycerol stock (produced later)
Inoculate CMV into LB medium with amicillin
- CMV (BBa_J52034) from 10.8.2010 inoculated into LB medium with ampicillin, as falsly inoculated in Kanamycin
Agarosegelelectrophoresis with digestions
->Protocol (11 Agarose gel electrophoresis)
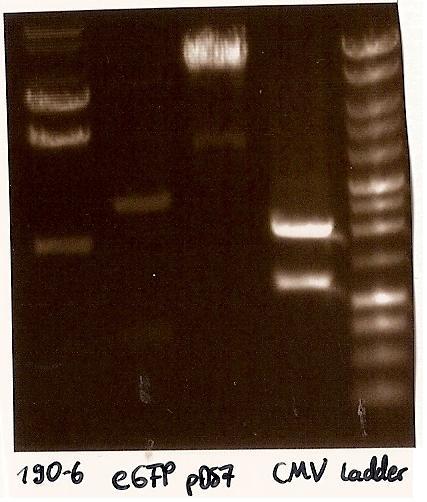
- Agarosegelelectrophoresis with the digestions (CMV, eGFP, pDS7, 190-6), 125V for 30 minutes and then for 20 minutes;
- expected DNA bands: 190-6 (4840bp, 1903bp), pDS7 (8027bp, 6bp), CMV (654 bp (Insert), 2079bp (Plasmid)), eGFP (720bp (Insert), 2750bp (Plasmid))
- Correct DNA bands for 190-6 (~4800bp, ~1900bp, ~6700bp (undigested plasmid)) and eGFP (~2000bp (Plasmid), ~750 bp (Insert)); CMV probably not digested (two bands; one probably normal, one supercoiled) and pDS7 not clear
Restriction digest from CMV and pDS7
-> Protocol (5 Restriction digest)
- Restriction digest from CMV (EcoR1, Pst1; 6µl DNA, buffer H) and pDS7 (EcoR1, Spe1; 2µl DNA, buffer B)
Agarosegelectrophoresis with digestions
->Protocol (11 Agarose gel electrophoresis)
- Agarosegelelectorphoresis for 30 minutes, 150V
- Expected DNA bands: CMV see above, pDS7 (3647bp, 3369bp, 1011bp, 6bp)
- false DNA bands CMV (~1200 bp, ~2000 bp) and pDS7 (~8000bp two bands, ~1100 bp); required to isolate a new colony for these two Plasmidextractions
Plated CMV on Ampicllin-Agar
- Plated the colony from CMV (BBa_J52034) for Plasmidextraction (Ampicillin), as falsly plated on Kanamycin
weekend
weekend
Planting colonies
- transfer 1 ml PhiC31o culture to new LB medium + Amp, 37°C
- pick up CMV and pDS7 colonies from plates and transfer to LB medium+Amp, 37°C
Plasmid Extraction of PhiC31o
->Protocol (4 Plasmid extraktion from cells)
- plasmid extraction of PhiC310
->27,5ng/µl DNA and second plasmid extraction of PhiC310 (i. o. to get more DNA); first eluation-step with first eluation-extraction
-> 60ng/µl DNA
Restriction digest
->Protocol (5 Restriction digest)
- restriction digest of PhiC310 with EcoR1 and Spe1
restriction digest in the thermo cycler (program "Verdau", see protocol)
Handling primers after arrival (1,2,3,4,5,6,11,12)
->Protocol (9 Handling primers)
PCR preparations
- 10mM dNTP mix made from 100 mM dATP, dGTP, dCTP, dTTP by taking 100µl of each and adding 600µl H 2 O
PCR 1 and 6
- PCR of the tet inducible CMV minimal promotor out of prevTRE (=PCR 1 with Primer 1 and 2) and SV40PA out of pcDNA3 (=PCR 6 with Primer 11 and 12)
->Protocol (10 PCR with Pfu)
Mixture:
Glycerolstock of PhiC31o
- Glycerolstock of the colony of PhiC31o for the plasmidextraction
Plate CMV and pDS7 colonies on Ampicillin-Agar
- colonies for plasmidextraction of CMV and pDS7 plated on Ampicillinplates
Plasmid Extraction of CMV and pDS7
- plasmidextraction of CMV (2,5ng/µl) and pDS7 (10ng/µl) the A260/A280 value was 1.333, which means that it was 90% Protein and only 10% DNA (should be 1,8); new plasmidextraction needed
new overnight cultures of CMV and pDS7 for a new plasmidextraction made
Agarose gel electrophoresis
-> Protocol (11 Agarose gel electrophoresis)
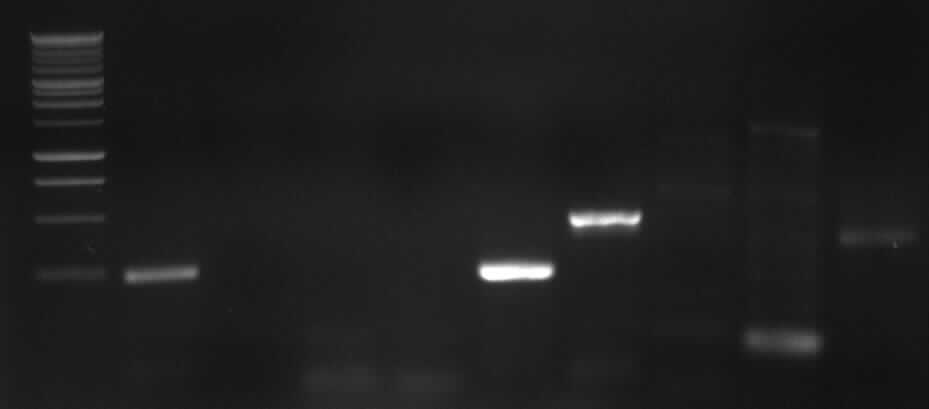
- Agarose gel electrophoresis of the restriction digest of PhiC31o and PCR 1 and 6
- the right bands found for PhiC31o (~2900,~2400,~250)
- the right band found for PCR1 (~450)
- no band found for PCR6; new electrophoresis needed with more DNA loaded
- new agarose gel electrophoresis from PCR6 with 5µl DNA instead of 3µl (image not yet shown)
- the right band found for PCR6 (~200)
New overnight cultures of CMV and pDS7
- the overnight colonies didn't grow; new colonies (CMV and pDS7) picked from plate and inoculated in LB Ampicillin
PCR purification of PCR 1 and 6
-> Protocol (12 Gel extraction or PCR Clean up)
- DNA concentration of the PCR 1 and 6 products measured: PCR1: 410ng/µl (A260/A280=1.253) PCR6: 568ng/µl (A260/A280=1.275)
- PCR Purification with Promega Kit
-> PCR1: 230ng/µl (A260/A280=1.769)
-> PCR6: 37.5ng/µl (A260/A280=1.667)
Plasmid Extraction of CMV and pDS7
-> Protocol (4 Plasmid extraction from cells)
- Plasmid extraction of CMV (97.5ng/µl; A260/A280=1.857) and pDS7 (212ng/µl; A260/A280=1.848)
Restriction digestion
-> Protocol (5 Restriction digest)
- Restriction digestion of CMV (EcoR1 + Pst1; 10µl DNA, buffer H) and pDS7 (EcoR1 + Spe1; 5µl DNA, buffer B)
-> expected DNA bands: CMV: 2079bp (plasmid) + 654bp (Insert); pDS7: 7022bp + 1011bp
Agarose Gel electrophoresis of digested CMV and pDS7
-> Protocol (11 Agarorse gel electrophoresis)
-> right DNA bands for pDS7 (~7000bp, ~1000bp)
-> false DNA bands for CMV
- Starting PCR 2a and 2b (replication and mutagenesis of pDS7): 3 µl DNA and 50°C Annealing Temperatur (other same as 8-16-2010)
Agarose gel electrophoresis of PCR 2a and 2b
-> Protocol (11 Agarose gel electrophoresis)
(150V, 30min)
-> the right bands for PCR2a (~300bp) and PCR2b (~700bp)
- New agarose gel electrophoresis with all of the PCR product for gel extraction (150V, 30min)
Gel extraction of the DNA from PCR2a and PCR2b
-> Protocol (12 Gel extraction or PCR Clean up)
- DNA concentration measured; problem with nanodrop as too low concentration; lyophille used to reduce volume
- DNA concentration measured again: PCR2a: 70ng/µl A260/A280=1.647; PCR2b: 45ng/µl A260/A280=1.5
PCR 3 (joining PCR of 2a and 2b)
- PCR3 (the joining PCR of PCR2a and 2b; Joining of the TEVrecogn-N-Degron-SF3 part) done: 1.3 µl of PCR2a and 4.7 µl of PCR2b makes 300ng of a 1:1 solution of both to be joined DNA parts. Annealing temperature: 50°C
-> Protocol (10 PCR with Pfu)
Agarose gel electrophoresis of PCR3
left column: marker; most right column: PCR3
-> Protocol: 11 Agarose gel electrophoresis (150V, 30min)
-> expected band: ~1000bp
-> false band: ~500bp
- probable reason: mini photometre was influenced by gel extraction chemicals, therefore it measured false DNA concentrations and false template masses were calculated
-> New 2a and 2b PCR
New PCR (2a and 2b)
-> Protocol: 10 PCR with Pfu
(see 8-18-2010, but 35,5µl water)
weekend
weekend
Agarose gel electrophoresis of PCR 2b
-> Protocol: 11 Agarose gel electrophoresis
- expected band: 700bp
-> no band shown on gel -> new PCR 2b
PCR 2b
- start PCR 2b with PCR 2b from 8-13-10 as template ( 1:20 and 1:100 diluted; 1µl)
-> Protocol: 10 PCR with Pfu
- annealing temperature: 50°C; amount of water: 37,5µl
Agarose gel electrophoresis of PCR 2b 1:20 and 1:100
-> Protocol: 11 Agarose gel electrophoresis
- expected bands: each ~ 700bp
- false bands: ~ 200bp
-> new PCR with 2ng, 5ng, 10ng template pDS7
gelextraktion
to do:
-gelextraktion von 2a und 2b, kit von frau gebhard
-wenn konzentrationen ok, PCR 3, sonst DNa fällen (gebhard)
-CMV PCR mit Biobrick primern
-sonst: Übernachtkultur(en?) von CMV ansetzen
-schauen, welche PCRs noch möglich sind
agarose gel electrophoresis of PCR 2b
- expected bands: right bands with 2ng and 5ng template (~700bp), no band with 10ng template
- CMV plasmid extraction:
- CMV restriction digest: EcoRI, PstI, buffer H
text
text
text
weekend
weekend
text
text
text
text
text
weekend
weekend
text
text
text
text
text
weekend
weekend
text
text
text
text
text
weekend
weekend
text
text
text
text
text
weekend
weekend
text
text
text
text
text
weekend
weekend


![]()
![]()







![]()
![]()

![]()
Apoptosis Notebook
Contents
Week Days
Monday
Tuesday
Wednesday
Thursday
Friday
Saturday
Sunday
31
8-02-2010
8-03-2010
8-04-2010
8-05-2010
8-06-2010
8-07-2010
8-08-2010
32
8-09-2010
8-10-2010
8-11-2010
8-12-2010
8-13-2010
8-14-2010
8-15-2010
33
8-16-2010
8-17-2010
8-18-2010
8-19-2010
8-20-2010
8-21-2010
8-22-2010
34
8-23-2010
8-24-2010
8-25-2010
8-26-2010
8-27-2010
8-28-2010
8-29-2010
35
8-30-2010
8-31-2010
9-01-2010
9-02-2010
9-03-2010
9-04-2010
9-05-2010
36
9-06-2010
9-07-2010
9-08-2010
9-09-2010
9-10-2010
9-11-2010
9-12-2010
37
9-13-2010
9-14-2010
9-15-2010
9-16-2010
9-17-2010
9-18-2010
9-19-2010
38
9-20-2010
9-21-2010
9-22-2010
9-23-2010
9-24-2010
9-25-2010
9-26-2010
39
9-27-2010
9-28-2010
9-29-2010
9-30-2010
10-01-2010
10-02-2010
10-03-2010
8-02-2010
8-03-2010
- test5
8-04-2010
header 1
header 2
header 3
row 1, cell 1
row 1, cell 2
row 1, cell 3
row 2, cell 1
row 2, cell 2
row 2, cell 3
8-05-2010
H2Oddes
10,3 µl
RE10 + Buffer H
2,0 µl
acetylated BSA
0,2 µl
DNA
6,0 µl
apple banana peaches
green yellow red
8-06-2010
8-07-2010
8-08-2010
test
8-09-2010
farbnummern für farbige schrift: http://html.nicole-wellinger.ch/hilfen/farbenverzeichnis.html
8-10-2010
Plasmid Isolation
8-11-2010
Prepared overnight culture of eGFP BBa_I714891
Prepared overnight culture of 190-6 and pDS7 and eGFP (BBa_I714891) in falcons
Restriction digest (Restriktionsverdau) of CMV-Promoter BBa_J52034 with EcoRI and PstI
H2Oddest, sterile
10,3 µl
RE10 + Buffer H
2,0 µl
acetylated BSA (18ng/µl)
0,2 µl
DNA (0,143µg/µl)
6,0 µl
Prepare new/fresh overnight culture of CMV-Promoter Biobrick: BBa_J52034
8-12-2010
8-13-2010
8-14-2010
8-15-2010
8-16-2010
H2Oddest, sterile
0 µl
Buffer B
2,0 µl
BSA (1:10)
2 µl
DNA (0,06µg/µl)
15,0 µl
EcoR1
0,5 µl
Spe1
0,5µl
pcDNA3 (0,6 µg/µl)
pTRERev (0,15µg/µl)
Primer
2*2,5µl (P1+P2)
" (P11+P12)
300ng template
0,5µl
2µl
10x Buffer Pfu
5µl
"
dNTP Mix
1µl
"
Pfu Polymerase (3u/µl)
0,5µl
"
H2O
40,5µl
39µl
summ
52,5µl
52,5µl
Programme:
Denaturation
95°C
2min
30 times:
Denaturation
95°C
1min
Annealing
45°C
30sec
Extension
73°C
2min
Final Extension
73°C
5min
Soak (end)
12°C
infinite
bacterial culture
800µl
Glycerol (50%)
500µl
8-17-2010


Agarose gel electrophoresis of (from left to right) PhiC31o, PCR1 and PCR6
Agarose gel electrophoresis of (from left to right) PhiC31o, PCR1 and PCR6 which shows that PCR1 is between 250 and 500 bp
8-18-2010
8-19-2010
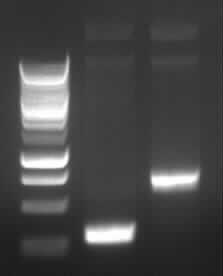
Agarose gel electrophoresis of (from left to right) PCR2a and PCR2b
8-20-2010
8-21-2010
8-22-2010
8-23-2010
PCR 2b with 2ng, 5ng, 10ng template pDS7
->
- wenn primer kommen:
8-24-2010
- PCR2b was gel extracted (with Qiagen gel extraction kit), 17.5 ng/µl a260/A280= 1.750
- PCR3: conducted again at 52°C annealing temperature
PCR2a
0.9 µl
PCR2b
0.6 µl
primer3
2.5 µl
primer6
2.5 µl
dNTPs
1 µl
Pfu
0.5 µl
10xbuffer
5 µl
H2O
37 µl
- agarose gel electrophoresis (150V, 25 min) of the CMV digest
-> bandes are wrong again ( ~ 1200bp, 2000bp)
8-25-2010
8-26-2010
8-27-2010
8-28-2010
8-29-2010
8-30-2010
8-31-2010
9-01-2010
9-02-2010
9-05-2010
9-04-2010
9-05-2010
9-06-2010
9-07-2010
9-08-2010
9-09-2010
9-10-2010
9-11-2010
9-12-2010
9-13-2010
9-14-2010
9-15-2010
9-16-2010
9-17-2010
9-18-2010
9-19-2010
9-20-2010
9-21-2010
9-22-2010
9-23-2010
9-24-2010
9-25-2010
9-26-2010
9-27-2010
9-28-2010
9-29-2010
9-30-2010
10-01-2010
10-02-2010
10-03-2010
![]()
![]()

 "
"