Team:Bielefeld-Germany/Project/Outlook
From 2010.igem.org
(→Pheochromocytoma) |
(→Diagnosis of pheochromocytoma and neuroblastoma (child tumors)) |
||
| Line 76: | Line 76: | ||
<img src="http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?t=l&cid=23615482" width="200"> | <img src="http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?t=l&cid=23615482" width="200"> | ||
<img src="http://pubchem.ncbi.nlm.nih.gov/image/img3d.cgi?cid=23615482" width="200"> | <img src="http://pubchem.ncbi.nlm.nih.gov/image/img3d.cgi?cid=23615482" width="200"> | ||
| - | <h3>Vanilmandelic | + | <h3>Vanilmandelic acid</h3> |
<a href="http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=23615482&loc=ec_rcs" target="_blank">see pubchem datasheet</a> | <a href="http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=23615482&loc=ec_rcs" target="_blank">see pubchem datasheet</a> | ||
</div> | </div> | ||
Revision as of 19:30, 27 October 2010
Contents |
The techniques’ potential
In our project we modulated the virA-gene from A. tumefaciens, which is encoding a receptor for the phyto hormone acetosyringone, via error prone PCR. Slight mutations may alter the receptors conformation or its binding site. Both of these can cause shifts in specificity and sensitivity. This way we tried to create a new receptor, which is capable of sensing capsaicin. The chemical formulas of capsaicin and acetosyringone are quite similar and there are other molecules of interest, that have some of the necessary properties, too. Thus, we suggest that it is possible to modulate VirA receptors in order to make them sense a variety of highly relevant compounds.
Further Possible Substrates
Here we are listing some other candidates our sensing system may be applied to. All of them have similiar molecular structures as the VirA ligand natural inducor acetosyringone. In the following we will present some of the most relevant compounds other than capsaicin, that may be upcoming targets of our research.
All of the following images of the compounds molecular structures are linked to [http://pubchem.ncbi.nlm.nih.gov/ The PubChem Project].
Diagnosis of pheochromocytoma and neuroblastoma (child tumors)
Pheochromocytoma
Pheochromocytoma is a rare endocrine tumor originating in the medulla of adrenal glands, localized on top of the kidney. The adrenal glands produce several catecholamines, to which metanephrine and dopamine belong. These hormones regulate stress responses, heart rate and blood pressure. In patients with pheochromocytoma these hormones are released excessively, potentially causing increased heart rate and blood pressure. Pheochromocytoma may become life threatening when not recognized and treated [http://pheopara.nichd.nih.gov/ (Pheochromocytoma and Paraganglioma website at the NIH)].
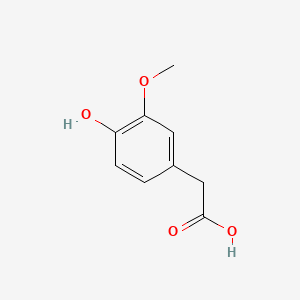
These hormones can be quantified in urine and thus are important compounds of pheochromocytoma diagnostics. [http://jcem.endojournals.org/cgi/content/abstract/92/12/4602 Boyle et al. (2007)] compare different accuracies of diagnostic measures for the tumor and name urinary free metanephrine HPLC-measurement as the most sensitive and specific. Homovanillic acid and vanillyl mandelic acid are measured via HPLC aswell and are indicators for the same tumor.
Neuroblastoma
"Neuroblastoma is the most common extracranial solid tumor in infancy. It is an embryonal malignancy of the sympathetic nervous system arising from neuroblasts (pluripotent sympathetic cells). In the developing embryo, these cells invaginate, migrate along the neuraxis, and populate the sympathetic ganglia, adrenal medulla, and other sites. The pattern of distribution of these cells correlates with the sites of primary disease presentation." ([http://emedicine.medscape.com/article/988284-overview Lacayo, Davis 2010])
Patients with high-risk disease still have very poor outcomes despite intensive therapy.
"More than 90% of patients have elevated homovanillic acid (HVA) and/or vanillylmandelic acid (VMA) levels detectable in urine."
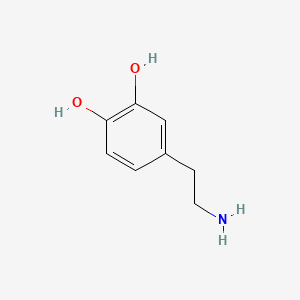
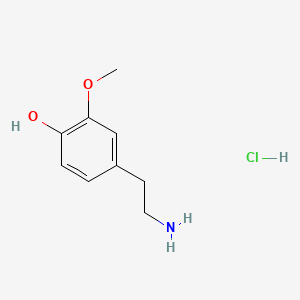
3-Methoxytyramine
[http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0013452 Sotnikova et al. (2010)] suggest, that 3-Methoxytyramine (3-MT) plays an important role as a neuromodulator. 3-MT is a metabolite of Dopamine and is reported to be a potential indicator for dopamine-dependent diseases like the parkinson disease, schizophrenia and dyskinesia. 3-Methoxytyramin as metabolic product of the hormon dopamine can be detected in human urine ([http://edoc.hu-berlin.de/oa/degruyter/cclm.1971.9.6.478.pdf Knoll et al., 1971]). Dopamine is known as happiness hormone found in increased levels after taking stimulating substances such as cocaine or amphetamines. The latter is one of the worldwide most used substitutes in sportive competition as doping. Increased levels of 3-MT analyzed in urine taken from sportives can indicate to intake of stimulating substances ([http://journals.lww.com/acsm-msse/Abstract/1980/21000/The_effect_of_amphetamines_on_selected.13.aspx CHANDLER, JOE V. and STEVEN N BLAIR, 1980]).
Dopamine
Even though being referred to as "happiness hormone", Dopamine and its receptors are said to play key roles in numerous phsychic disorders and drug addiction. For many years now, the Dopamine hypothesis of Schizophrenia exists and evolves. It states that many symptoms of Schizophrenia correlate with a hyperactive disturbed dopaminergic signal transduction. [http://schizophreniabulletin.oxfordjournals.org/content/early/2009/03/26/schbul.sbp006.abstract Howes and Kapur (2009)] as well as [http://jop.sagepub.com/content/21/4/440.short Stone et al. (2007)] reviewed the hypothesis and its evolution.
Dopamin is strongly connected to reward mechnims in the brain. Hence it is involved in reward-related learning, behavior and evaluation of rewarding outcomes [http://www.nbb.cornell.edu/neurobio/linster/BioNB420/pdfs/martin_soelch_etal_2001.pdf Martin-Soelch et al. (2001)]. Concerning drug addiction, Dopamine and the brains reward system are of special interest in research [http://dx.doi.org/10.1016/j.brainresrev.2004.12.033 Heidbreder et al. (2005)]. Further Dopamine plays a key role in deseases, e.g. the parkinson desease [http://www.nejm.org/doi/pdf/10.1056/NEJM198804073181402 Kish et al. (1988)].
Picric acid
Nitroaromatic compounds, such as Picrid acid (2,4,6-trinitrophenol), can be used as dyes, explosives, pesticides and energy sources for bacterial growth. The industrial use ([http://www.springerlink.com/content/q82535h870021540/fulltext.pdf Rajan, J 1996]) of picric acid contaminates ground water. Additional picric acid is as nitritited aromatic compound in a dry state a potential explosive ([http://search.barnesandnoble.com/Explosives-Engineering/Paul-W-Cooper/e/9780471186366 Cooper PW, 1997]; [http://onlinelibrary.wiley.com/doi/10.1002/1521-3773%2820010601%2940:11%3C2104::AID-ANIE2104%3E3.0.CO;2-%23/full Sohn H, 2001]). Picric Acid is still a problem by unfound warefare. Moreover there are often accidents in german schools due to dry picric acid [http://www.spiegel.de/schulspiegel/wissen/0,1518,572376,00.html spiegel-online (08/18/2010)]. Existing detection methods are time consuming and even more cost intensive, so that a high selective and sensitiv application by microbial screening would be desirable. We decided not to work with picric acid because of its high [http://www.jtbaker.com/msds/englishhtml/p4556.htm explosive potential].
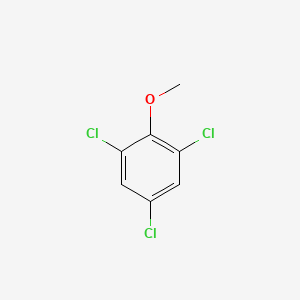
Trichloranisol
Trichloranisol (2,4,6 Trichloranisol) ist assumingly a compound causing cork taint in wine. The origin is supposed to find in pestizid use for cork oak tree. Further contamination can be found in the barrels used for wine maturation ([http://pubs.acs.org/doi/pdf/10.1021/jf00110a037 Buser et al., 1982)]. A sommelier could use a pre test system for preventing to serve a contaminated high class wine to a costumer. Our Test system could be adaptable for Trichloranisol, adding, beside Capsaicin, an application in the field of indulgence.
Summary
The VirA receptor is a great starting point for modulations, in order to address a wide variety of highly relevant compounds. By contructing a Vir-gene based sensing system we present a proof of concept for a general microbial sensing system adaptable for various other compounds. Since the basic sensing and reporting system was successfully established in E. coli, the next essential steps are to improve the mutagenesis strategy for the receptor, and finally the screening for each candidate compound.
Using a set of sensitivity tuners, the reporter output may then be optimized. Furthermore, the speed of the systems are expected to require refinement.
As a result, the MARSS has great potential to deliver rapid biological tests for medical diagnosis, drug tests for e.g. doping conctrols, analysis of food for e.g. allergy triggering compounds and soil contaminating substances such as picric acid.
References
- Boyle J, Davidson DF, Perry CG and Connell JMC, Comparison of Diagnostic Accuracy of Urinary Free Metanephrines, Vanillyl Mandelic Acid, and Catecholamines and Plasma Catecholamines for Diagnosis of Pheochromocytoma ,Journal of Clinical Endocrinology & Metabolism, Vol. 92, No. 12, pp. 4602-4608
- Chandler JV and Blair SN (1980), The effect of amphetamines on selected physiological components related to athletic success. Med. Sci. Sports Exercise, Vol. 12, No. 1, pp. 65-69
- Cooper PW (1997), Explosives Engineering, Edition 1, Wiley, John & Sons.
- Eunice Kennedy Shriver National Institute of Child Health and Human Development, Pheochromocytoma and Paragangliooma, 0-CH-0093
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR Jr. (2005), The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence., Brain Research Reviews, Vol. 49, pp. 77 – 105
- Howes OD and Kapur S (2009), The Dopamine Hypothesis of Schizophrenia: Version III—The Final Common Pathway, Schizophrenia Bulletin, Vol. 35, No. 3, pp.549-62
- Kish JS, Shannak K, Hornykiewicz O (1988), Uneven Pattern of Dopamine Loss in the Striatum of Patients with Idiopathic Parkinson's Disease, New England Journal of Medicine Vol. 318, No. 14, pp. 876
- Knoll E, Wisser H, Stamm D (1971), in Verfahren zur Bestimmung der 3-Methoxy-4-hydroxy-phenylessigsäure (Homovanillinsäure) im Harn durch in situ Remissionsmessung nach dünnschichtchromatographischer Trennung, Z. klin. Chem. u. klin. Biochem., 1971
- Lacayo NJ (2010), Neuroblastoma, eMedicine from webMD
- Martin-Soelch C, Leenders KL, Chevalley AF, Missimer J, Künig G, Magyar S, Mino A, Schultz W (2001), Reward mechanisms in the brain and their role in dependence: evidence from neurophysiological and neuroimaging studies, Brain Research Reviews Vol. 36, pp. 139-149
- Rajan J,Valli K, Perkins RE, Sariaslani FS, Barns FM, Reysenbach A-L,Rehm S, Ehringer M and Pace NR (1996), Mineralization of 2,4,6-trinitrophenol (picric acid): characterization and phylogenetic identification of microbial strains Journal of Industrial Microbiology, 16, 319-324
- Sohn H, Calhoun RM, Sailor MJ, Trogler WC (2001), Detection of TNT and Picric Acid on Surfaces and in Seawater by Using Photoluminescent Polysiloles, Angewandte Chemie, Vol.40, pp.2104–2105
- Sotnikova TD, Beaulieu J-M, Espinoza S, Masri B, Zhang X, Salahpour A, Barak LS, Caron MG, Gainetdinov RR (2010), The Dopamine Metabolite 3-Methoxytyramine Is a Neuromodulator, PloSONE
- Stone JM, Morrison PD, Pilowsky LS (2007), Review: Glutamate and dopamine dysregulation in schizophrenia — a synthesis and selective review, Journal of Psychopharmacology, Vol. 21, No. 4, pp. 440-452
weblinks:
- http://pubchem.ncbi.nlm.nih.gov/
- Padtberg C "Das Zeug gehört nicht an Schulen" (08/18/2010), spiegel-online
- [http://www.jtbaker.com/msds/englishhtml/p4556.htm picric acid safety note]
 "
"