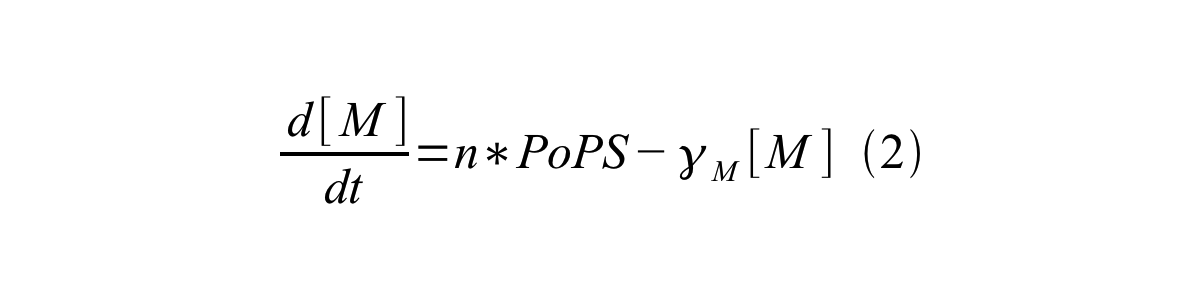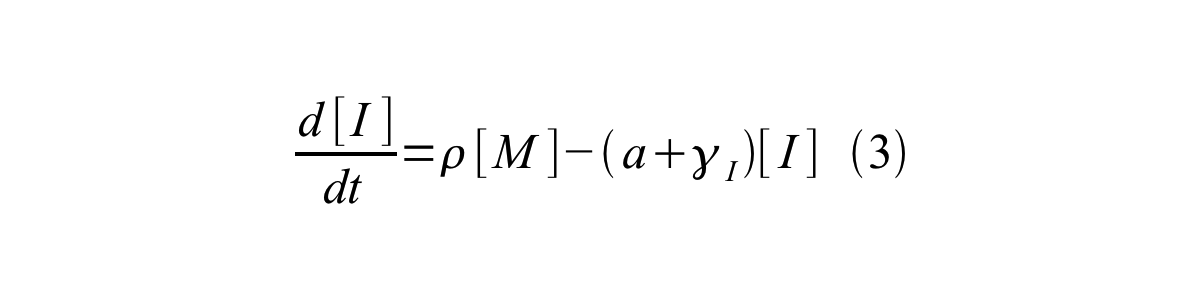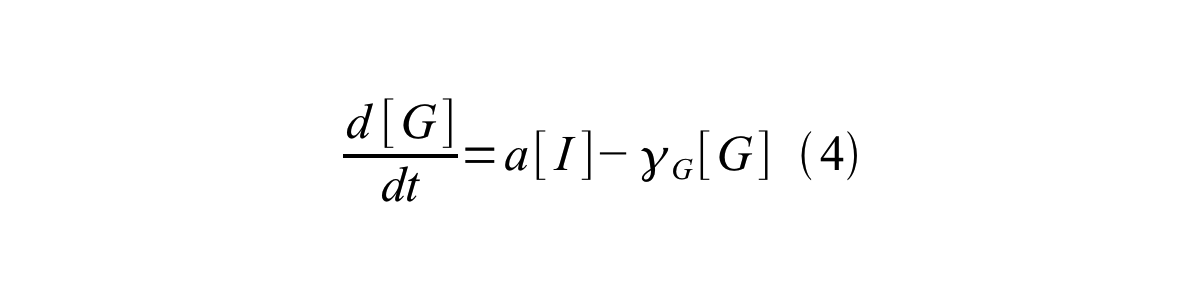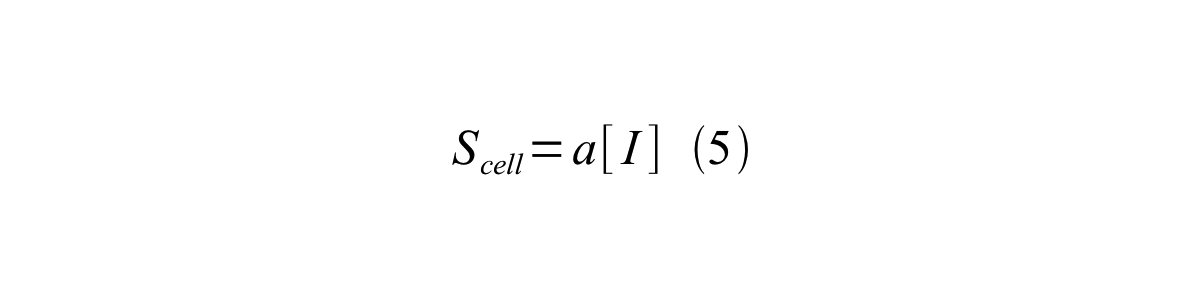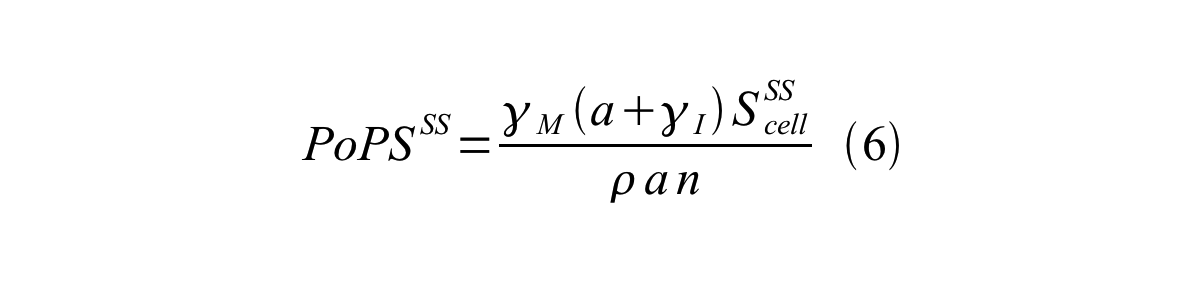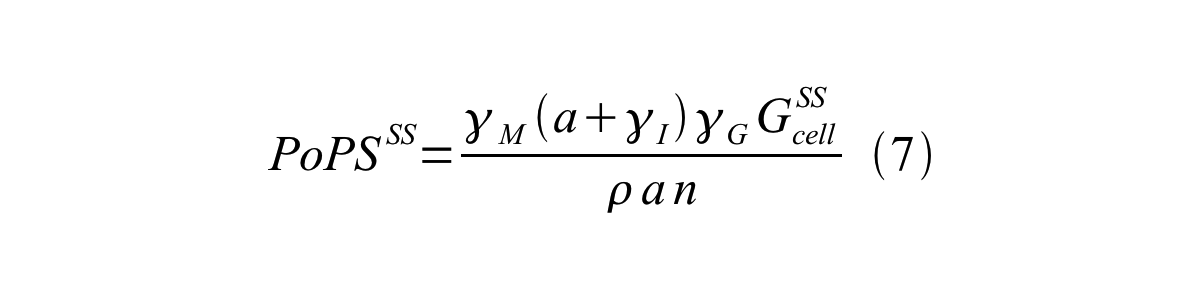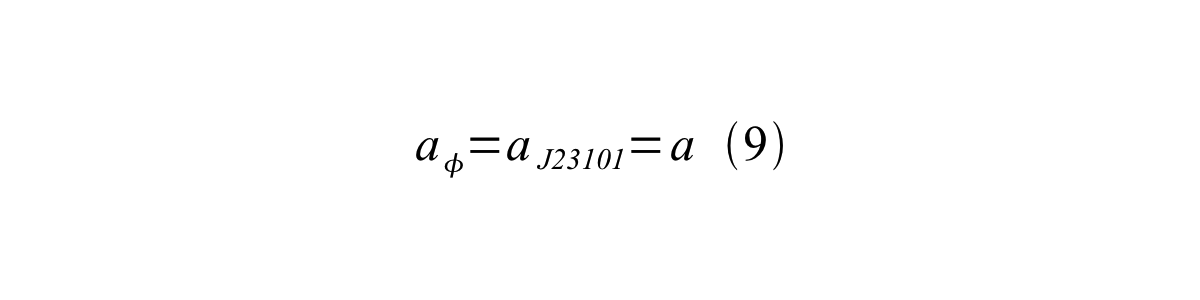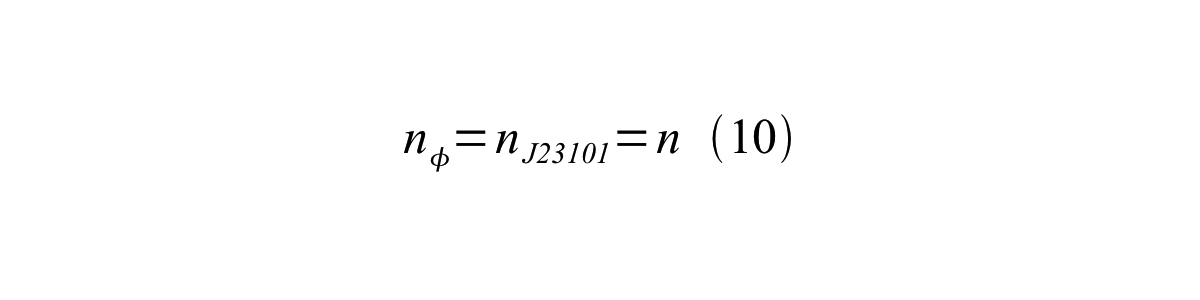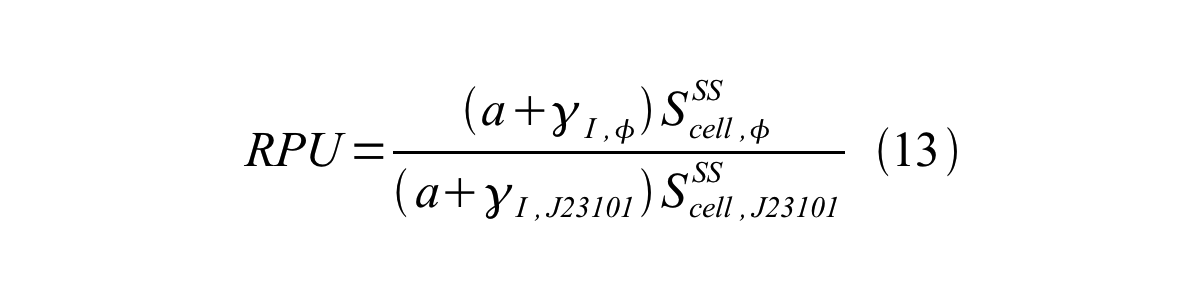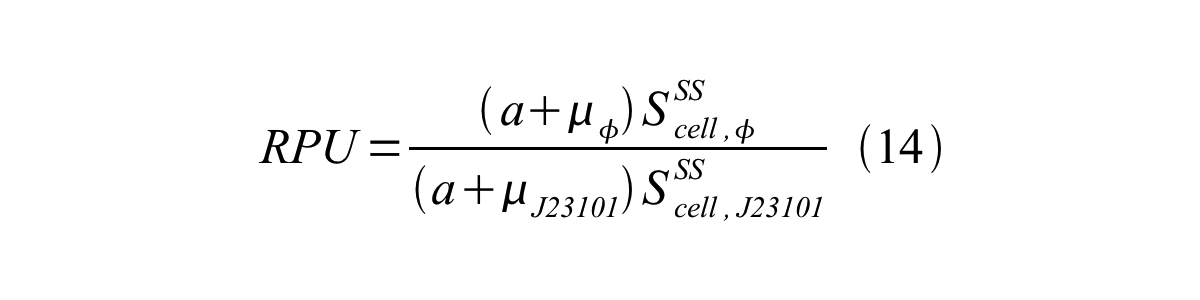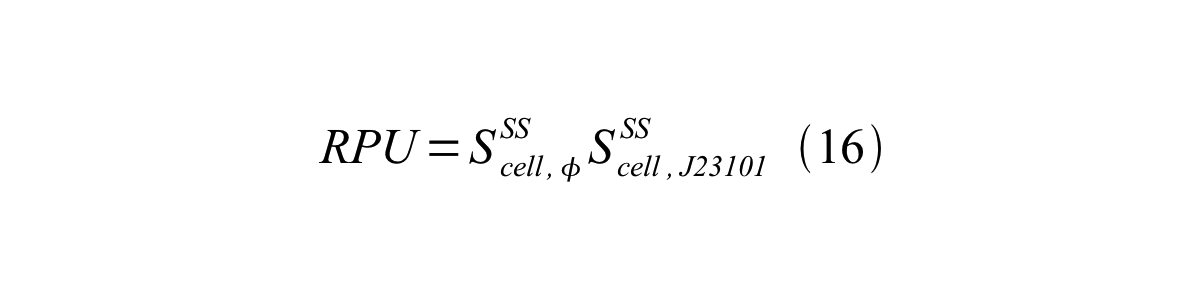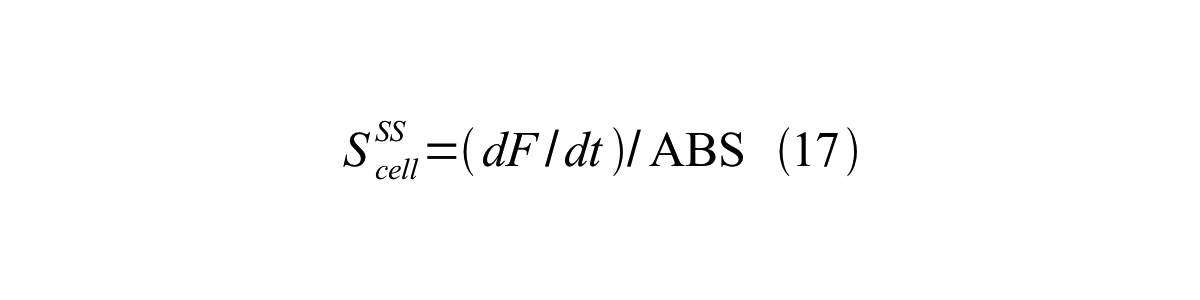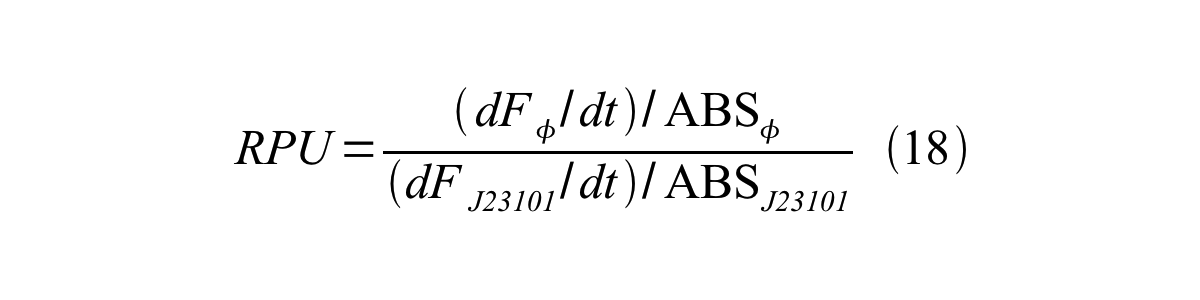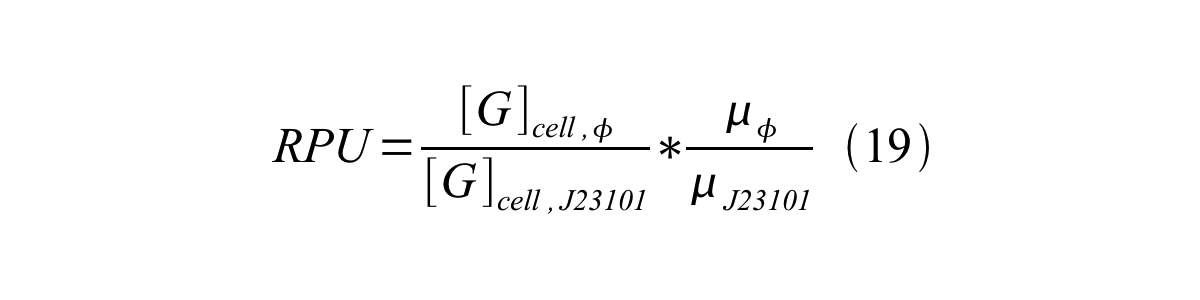Team:Kyoto/LearnMore
From 2010.igem.org
(→RPU (Relative Promoter Unit)) |
|||
| Line 1: | Line 1: | ||
{{:Team:Kyoto/Header}} | {{:Team:Kyoto/Header}} | ||
| - | == | + | ==LearnMore== |
| - | == | + | ===Lysis Cassette=== |
| - | ==RPU (Relative Promoter Unit)== | + | ===Sam7 Mutation=== |
| - | ===Overview=== | + | |
| + | ===RPU (Relative Promoter Unit)=== | ||
| + | ====Overview==== | ||
Though BioBrick parts are standardized in terms of how individual parts are physically assembled into multi-component systems, many of them are not standardized in the point of characterization. | Though BioBrick parts are standardized in terms of how individual parts are physically assembled into multi-component systems, many of them are not standardized in the point of characterization. | ||
| - | Unfortunately, it is known that the absolute activity of BioBrick promoters varies across experimental conditions and measurement instruments [[# | + | Unfortunately, it is known that the absolute activity of BioBrick promoters varies across experimental conditions and measurement instruments <sup>[[#ref1|[1]]]</sup>. However, Kelly et al. found that this variation could be reduced by measuring the activity of promoters relative to <partinfo>J23101</partinfo> which is a promoter as an in vivo reference standard for promoter activity by ~50%. They defined a Relative Promoter Units (RPU) in order to report promoter characterization data in compatible units. In order to measure RPUs of promoters, the synthesis rate of Green Fluorescent Protein (GFP) encoded by mRNA transcribed from each promoter is observed. |
RPU is defined by equation 1: | RPU is defined by equation 1: | ||
| - | [[Image:KyotoPromoterActivity001.png| | + | [[Image:KyotoPromoterActivity001.png|600px|center]] |
| - | ===Derivation=== | + | ====Derivation==== |
PoPS (Polymerase Per Second) is the unit of absolute “promoter activity”. It is defined as the number of RNA polymerase molecules that pass by the final base pair of the promoter and continue along DNA as an elongation complex. PoPS<sup>SS</sup> is PoPS at the steady state of following system (d[M]/dt=0, d[I]/dt=0, d[G]/dt=0: | PoPS (Polymerase Per Second) is the unit of absolute “promoter activity”. It is defined as the number of RNA polymerase molecules that pass by the final base pair of the promoter and continue along DNA as an elongation complex. PoPS<sup>SS</sup> is PoPS at the steady state of following system (d[M]/dt=0, d[I]/dt=0, d[G]/dt=0: | ||
| - | [[Image:KyotoPromoterActivity002.png| | + | [[Image:KyotoPromoterActivity002.png|600px|center]] |
| - | [[Image:KyotoPromoterActivity003.png| | + | [[Image:KyotoPromoterActivity003.png|600px|center]] |
| - | [[Image:KyotoPromoterActivity004.png| | + | [[Image:KyotoPromoterActivity004.png|600px|center]] |
Where: | Where: | ||
| Line 30: | Line 32: | ||
By combining equation 2, 3, 4 and 5 at the steady state, equation 6 and 7 are obtained: | By combining equation 2, 3, 4 and 5 at the steady state, equation 6 and 7 are obtained: | ||
| - | [[Image:KyotoPromoterActivity005.png| | + | [[Image:KyotoPromoterActivity005.png|600px|center]] |
Here Scell is define as the per cell mature GFP synthesis term. | Here Scell is define as the per cell mature GFP synthesis term. | ||
| - | [[Image:KyotoPromoterActivity006.png| | + | [[Image:KyotoPromoterActivity006.png|600px|center]] |
| - | [[Image:KyotoPromoterActivity007.png| | + | [[Image:KyotoPromoterActivity007.png|600px|center]] |
Here G<sub>cell</sub> is define as the per cell mature GFP synthesis term. | Here G<sub>cell</sub> is define as the per cell mature GFP synthesis term. | ||
| Line 40: | Line 42: | ||
From equation 1 and 6 RPU is described as equation8. | From equation 1 and 6 RPU is described as equation8. | ||
| - | [[Image:KyotoPromoterActivity008.png| | + | [[Image:KyotoPromoterActivity008.png|600px|center]] |
If the test promoter φ and the reference standard promoter are measured under the same culture conditions and both promoters are carried on the same backbone plasmid, following equations are approved. | If the test promoter φ and the reference standard promoter are measured under the same culture conditions and both promoters are carried on the same backbone plasmid, following equations are approved. | ||
| - | [[Image:KyotoPromoterActivity009.png| | + | [[Image:KyotoPromoterActivity009.png|600px|center]] |
| - | [[Image:KyotoPromoterActivity010.png| | + | [[Image:KyotoPromoterActivity010.png|600px|center]] |
| - | [[Image:KyotoPromoterActivity011.png| | + | [[Image:KyotoPromoterActivity011.png|600px|center]] |
| - | [[Image:KyotoPromoterActivity012.png| | + | [[Image:KyotoPromoterActivity012.png|600px|center]] |
From equation 8, 9, 10, 11, and 12, equation 13 is accomplished. | From equation 8, 9, 10, 11, and 12, equation 13 is accomplished. | ||
| - | [[Image:KyotoPromoterActivity013.png| | + | [[Image:KyotoPromoterActivity013.png|600px|center]] |
In addition, immature GFP is stable so that protein degradation is negligible compared to dilution due to cell growth (γ<sub>I</sub>, φ=μ<sub>φ</sub> and γ<sub>I,,J23101</sub>=μ<sub>J23101</sub>, where μis the cellular growth rate). Therefore, equation 13 can be modified to equation 14: | In addition, immature GFP is stable so that protein degradation is negligible compared to dilution due to cell growth (γ<sub>I</sub>, φ=μ<sub>φ</sub> and γ<sub>I,,J23101</sub>=μ<sub>J23101</sub>, where μis the cellular growth rate). Therefore, equation 13 can be modified to equation 14: | ||
| - | [[Image:KyotoPromoterActivity014.png| | + | [[Image:KyotoPromoterActivity014.png|600px|center]] |
And if the growth rates of both strains are almost same, equation 15 is approved. | And if the growth rates of both strains are almost same, equation 15 is approved. | ||
| - | [[Image:KyotoPromoterActivity015.png| | + | [[Image:KyotoPromoterActivity015.png|600px|center]] |
Accordingly, we can make equation 16 from equation15. | Accordingly, we can make equation 16 from equation15. | ||
| - | [[Image:KyotoPromoterActivity016.png| | + | [[Image:KyotoPromoterActivity016.png|600px|center]] |
S<sub>cell</sub><sup>SS</sup> can be described by equation17: | S<sub>cell</sub><sup>SS</sup> can be described by equation17: | ||
| - | [[Image:KyotoPromoterActivity017.png| | + | [[Image:KyotoPromoterActivity017.png|600px|center]] |
Therefore, RPU is described by equation 18: | Therefore, RPU is described by equation 18: | ||
| - | [[Image:KyotoPromoterActivity018.png| | + | [[Image:KyotoPromoterActivity018.png|600px|center]] |
We can also establish equation20 from equation1, 6, 9, 10, 11 and 12. | We can also establish equation20 from equation1, 6, 9, 10, 11 and 12. | ||
| - | [[Image:KyotoPromoterActivity019.png| | + | [[Image:KyotoPromoterActivity019.png|600px|center]] |
RPU can be calculated from equation 18 or equation 19. Therefore, we have to observe GFP synthesis rate and ABS (OD600) of both strains or to measure GFP concentration per cell and growth rate of both strains to measure RPU. | RPU can be calculated from equation 18 or equation 19. Therefore, we have to observe GFP synthesis rate and ABS (OD600) of both strains or to measure GFP concentration per cell and growth rate of both strains to measure RPU. | ||
| - | ===Note=== | + | ====Note==== |
To see how to measure RPU practically, go [[Team:Kyoto/Protocols#Measurement|Protocols]] | To see how to measure RPU practically, go [[Team:Kyoto/Protocols#Measurement|Protocols]] | ||
| - | ===Reference=== | + | ====Reference==== |
<div class="reference"> | <div class="reference"> | ||
| - | = | + | #<a name="ref1"></a> [http://www.ncbi.nlm.nih.gov/pubmed/19298678 PMID: 19298678] Kelly JR, Rubin AJ, Davis JH, Ajo-Franklin CM, Cumbers J, Czar MJ, de Mora K, Glieberman AL, Monie DD, Endy D., ''Measuring the activity of BioBrick promoters using an in vivo reference standard.'', J Biol Eng. 2009 Mar 20;3:4. |
| - | + | ||
</div> | </div> | ||
---- | ---- | ||
Revision as of 17:38, 21 October 2010
Contents |
LearnMore
Lysis Cassette
Sam7 Mutation
RPU (Relative Promoter Unit)
Overview
Though BioBrick parts are standardized in terms of how individual parts are physically assembled into multi-component systems, many of them are not standardized in the point of characterization.
Unfortunately, it is known that the absolute activity of BioBrick promoters varies across experimental conditions and measurement instruments [1]. However, Kelly et al. found that this variation could be reduced by measuring the activity of promoters relative to <partinfo>J23101</partinfo> which is a promoter as an in vivo reference standard for promoter activity by ~50%. They defined a Relative Promoter Units (RPU) in order to report promoter characterization data in compatible units. In order to measure RPUs of promoters, the synthesis rate of Green Fluorescent Protein (GFP) encoded by mRNA transcribed from each promoter is observed.
RPU is defined by equation 1:
Derivation
PoPS (Polymerase Per Second) is the unit of absolute “promoter activity”. It is defined as the number of RNA polymerase molecules that pass by the final base pair of the promoter and continue along DNA as an elongation complex. PoPSSS is PoPS at the steady state of following system (d[M]/dt=0, d[I]/dt=0, d[G]/dt=0:
Where:
- [M] is the concentration of mRNA,
- [I] is the concentration of immature GFP,
- [G] is the concentration of mature GFP,
- γM is the mRNA degradation rate,
- a is the GFP maturation rate,
- γI is the degradation rate of immature GFP
- n is the copy number of the plasmid containing the promoter
- ρ is the rate of synthesis of immature GFP in absolute units of protein per second per mRNA.
By combining equation 2, 3, 4 and 5 at the steady state, equation 6 and 7 are obtained:
Here Scell is define as the per cell mature GFP synthesis term.
Here Gcell is define as the per cell mature GFP synthesis term.
From equation 1 and 6 RPU is described as equation8.
If the test promoter φ and the reference standard promoter are measured under the same culture conditions and both promoters are carried on the same backbone plasmid, following equations are approved.
From equation 8, 9, 10, 11, and 12, equation 13 is accomplished.
In addition, immature GFP is stable so that protein degradation is negligible compared to dilution due to cell growth (γI, φ=μφ and γI,,J23101=μJ23101, where μis the cellular growth rate). Therefore, equation 13 can be modified to equation 14:
And if the growth rates of both strains are almost same, equation 15 is approved.
Accordingly, we can make equation 16 from equation15.
ScellSS can be described by equation17:
Therefore, RPU is described by equation 18:
We can also establish equation20 from equation1, 6, 9, 10, 11 and 12.
RPU can be calculated from equation 18 or equation 19. Therefore, we have to observe GFP synthesis rate and ABS (OD600) of both strains or to measure GFP concentration per cell and growth rate of both strains to measure RPU.
Note
To see how to measure RPU practically, go Protocols
Reference
- <a name="ref1"></a> [http://www.ncbi.nlm.nih.gov/pubmed/19298678 PMID: 19298678] Kelly JR, Rubin AJ, Davis JH, Ajo-Franklin CM, Cumbers J, Czar MJ, de Mora K, Glieberman AL, Monie DD, Endy D., Measuring the activity of BioBrick promoters using an in vivo reference standard., J Biol Eng. 2009 Mar 20;3:4.
 "
"