Template:UNIPV-Pavia/Project/results/Self-cleaving affinity tags to easily purify proteins
From 2010.igem.org
PHB production
| PHA production in R. eutropha is achieved by the PhaCAB operon, which contains three genes, phbC, phbA and phbB, each encoding for an enzyme essential for the formation of polyhydroxybutyrate (PHB, a kind of polyhydroxyalkanoate) inclusions. In literature, the production of PHB has already been achieved in E. coli by incorporating the PhaCAB operon. The PHB granules produced by engineered E. coli can be used as an affinity matrix for Phasin-based affinity tags and the binding of the Phasin-tagged protein of interest can occur in vivo.
Several parts for PHB production are present in the Registry: in the past iGEM editions, the Duke (2008 and 2009), Hawaii (2008), Tsinghua (2008), Virginia (2008) teams worked on it and submitted a set of BioBrick parts for PHB synthesis. However, the complete phaCAB operon is not available in the Registry. Because a lot of work about PHB has already been done and because our short term goal was not to optimize the PHB granules productions, an existing engineered E. coli strain able to produce PHB was used as a proof-of-concept affinity matrix producer. This strain comes from the DSMZ public collection and is named DMSZ15372. With the following experiment we tested the ability of E. coli to effectively synthesize PHB granules. |
Methods
Preparation of samples for BioPlastic screening:
- Inoculum into LB+Amp medium of:
- DMSZ15372 E. coli strain (harbouring pBHR68 plasmid - PBHR68 from now on)
- <partinfo>BBa_B0032</partinfo>
- Cultures were left grow ON at +37°C, 220 rpm.
- Cultures of PBHR68 and <partinfo>BBa_B0032</partinfo> were diluted 1:100 in fresh LB+Amp and than prepared as follows:
- <partinfo>BBa_B0032</partinfo> with NOTHING added (negative control)
- PBHR68 with NOTHING added
- PBHR68 + 2% glycerol (a carbon source for bioplastic production)
- PBHR68 + 1mM IPTG (inducer for Plac promoter, expressing bioplastic enzymes)
- PBHR68 + 2% glycerol + 1mM IPTG
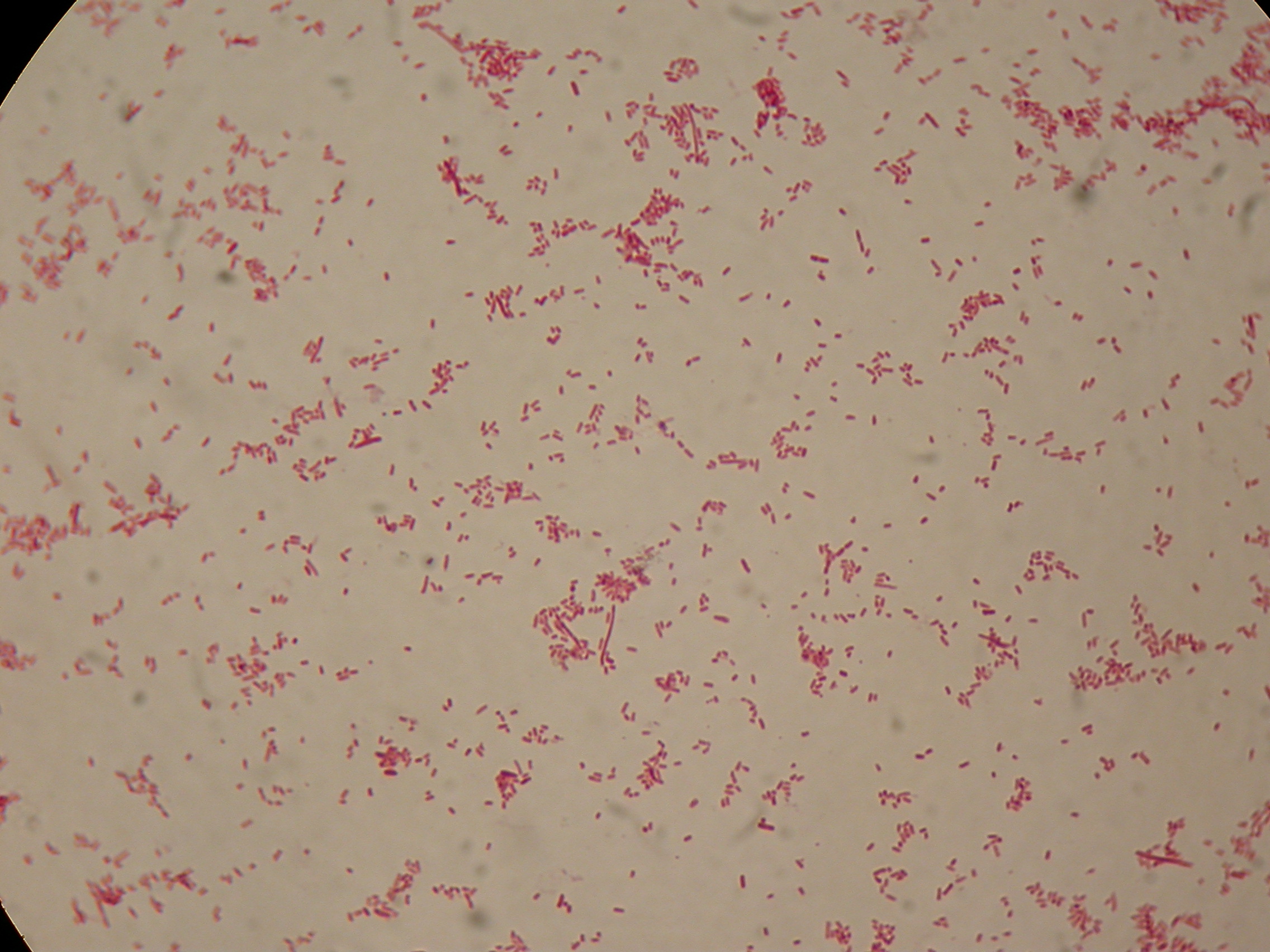
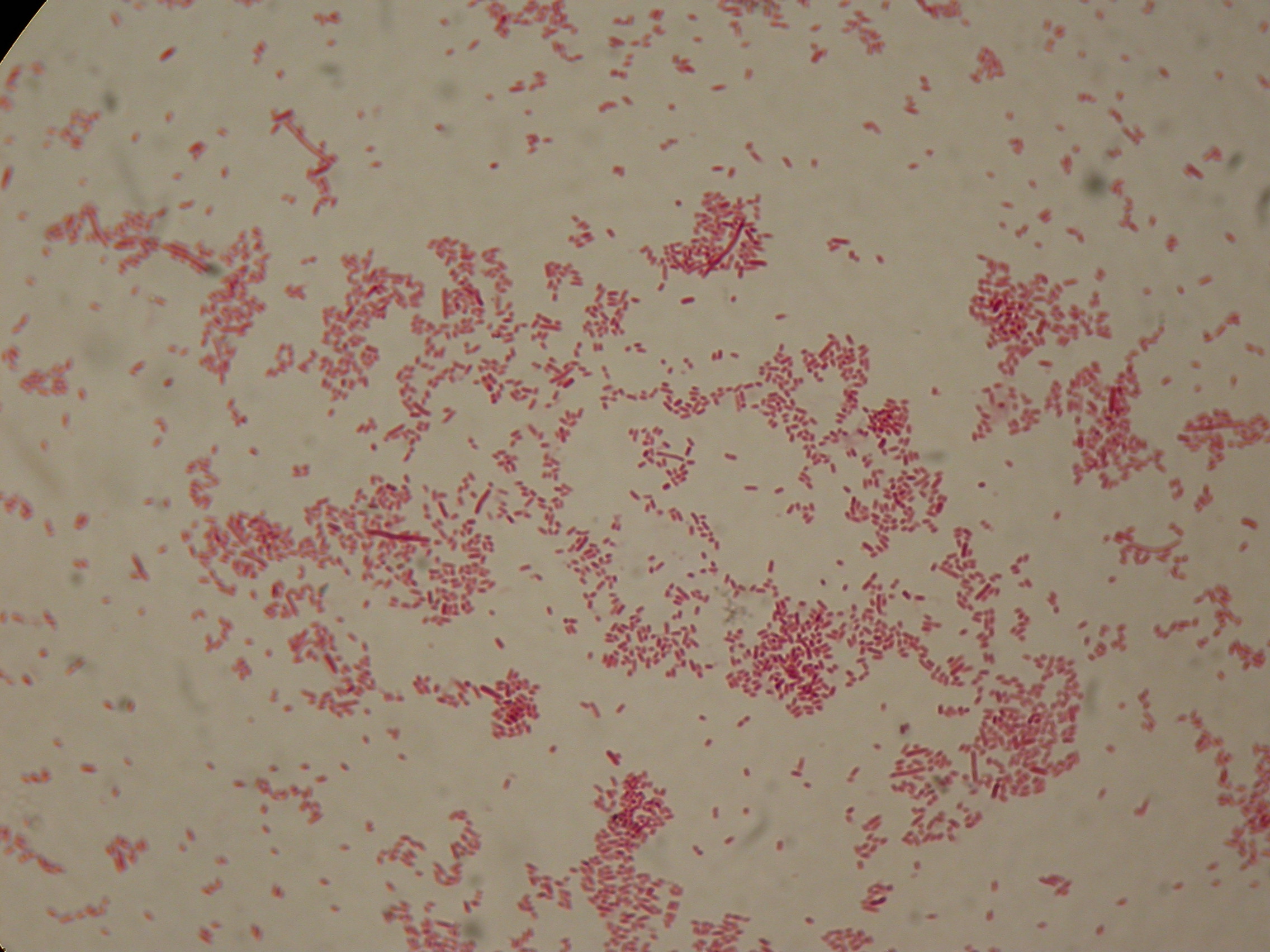
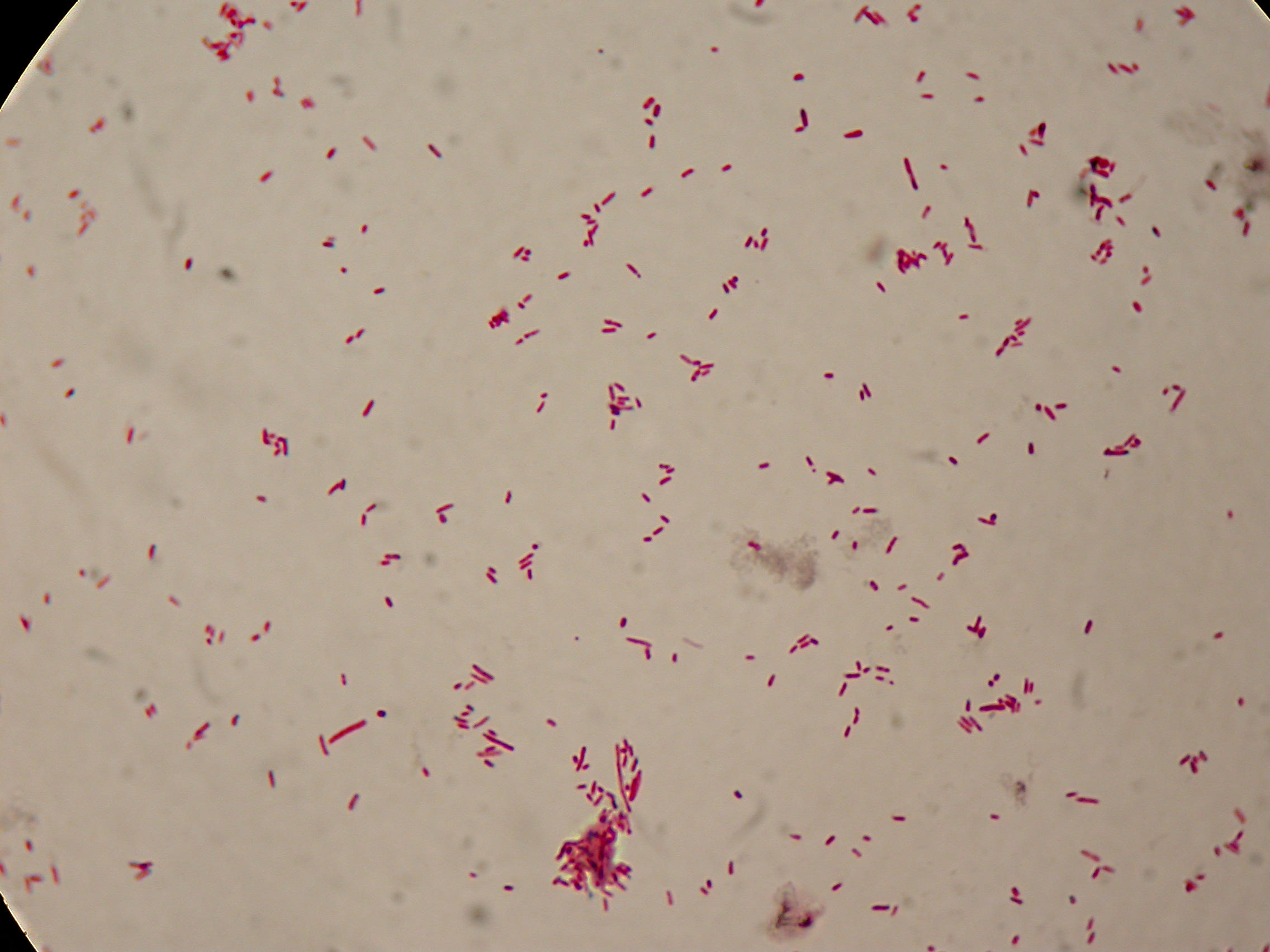
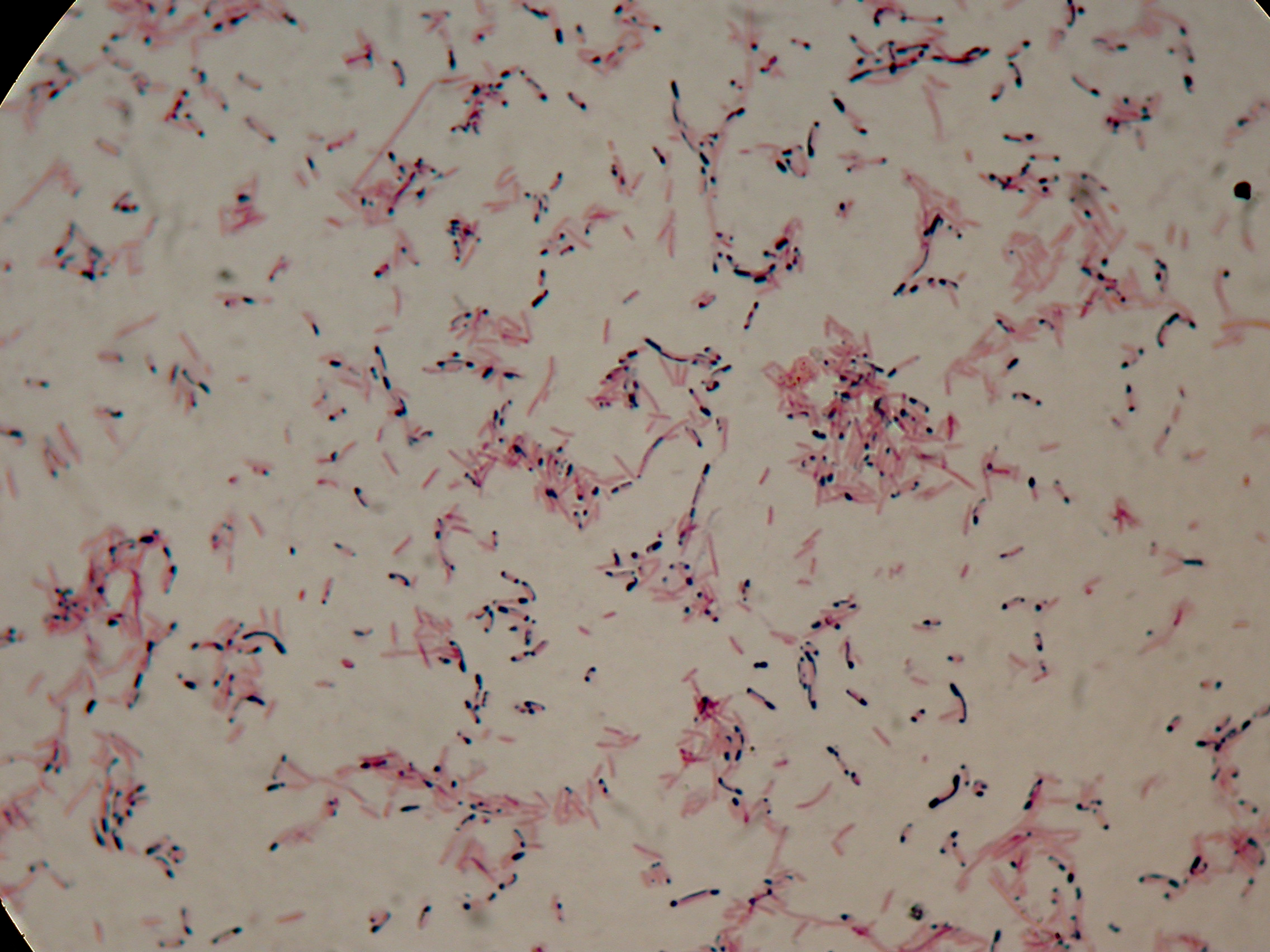
Sudan Black staining protocol was followed for microscope slider preparation after 5 and 30 hours' growth.
Results
5 hours'
Sudan Black staining protocol was performed on 70ul cultures and 5 microscope slides were prepared. The resulting images are shown here:
30 hours'
Sudan Black staining protocol was performed on 70ul cultures and 5 microscope slides were prepared. The resulting images are shown here:
Discussion
In images it's clearly possible to see that after 5 hours PBHR68 without any kind of addition is completely similar to negative control <partinfo>BBa_B0032</partinfo> and there is no trace of bioplastic granules production. In PBHR68 (E. coli cultures harbouring the pBHR68 plasmid) samples in which medium IPTG or glycerol was added, it's possible to see very small dark signs that could be identified as PHB granules in a few bacteria. In samples with addiction of both IPTG and glycerol bioplastic granules are clearly visible in many cells.
As expected after 30 hours negative control <partinfo>BBa_B0032</partinfo> does not show any trace of granules, while PBHR68 show bioplastic granules in each experimental condition. This shows that PHB can be produced both without the presence of the inducer (leakage activity of the plasmid) and glycerol (bacteria found another kind of carbon sources and used it for the synthesis).
This way the ability of E. coli to synthesize PolyHydroxyAlkanoates that can be used in the purification method presented in this project was demonstrated.
Fusion protein synthesis
These experiments were performed to check bacterial growth and GFP synthesis rate of the following constructs (and efficiency of inducible system of some of them) in order to verify right protein folding.
Costitutive promoter devices
Methods
Inoculum (into 5 ml LB+Amp) from glycerol stock of:
- <partinfo>BBa_K300086</partinfo>
- <partinfo>BBa_K300088</partinfo>
- <partinfo>BBa_K300090</partinfo>
- <partinfo>BBa_K300099</partinfo>
- <partinfo>BBa_K173000</partinfo> (positive control)
- <partinfo>BBa_B0031</partinfo> (negative control)
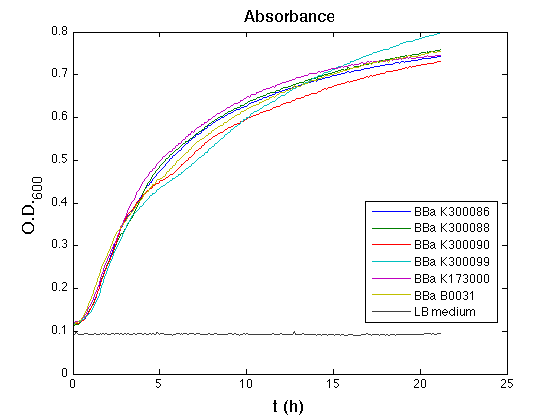
Cultures were grown ON at 37°C, 220 rpm.
The following day cultures were diluted 1:100 and let grow again for about five hours at 37°C, 220 rpm.
The optical density (O.D.) of each cell culture was than measured with TECAN Infinte F200. Samples were diluted in order to obtain the same O.D. equal to 0,02.
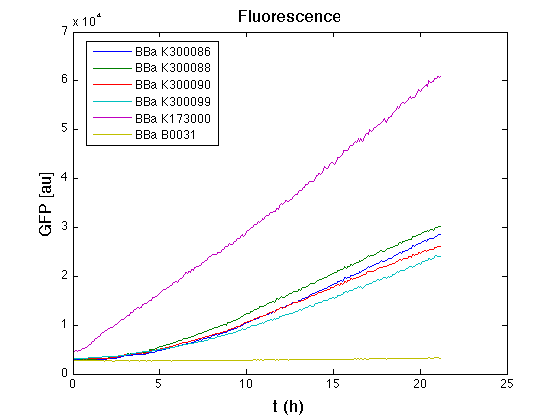
Then we performed a 21-hours' experiment with measurements of absorbance and green fluorescence every five minutes with TECAN Infinite F200; cultures were shaken for 15 seconds every five minutes. Each value shown below is the mean of three measurements, from GFP data that of a non-fluorescent culture (negative control) was subtracted.
Results
| Culture | Doubling time [min.] ± std error |
|---|---|
| <partinfo>BBa_K173000</partinfo> | 76.3336 ± 1.4362 |
| <partinfo>BBa_K300086</partinfo> | 73.6685 ± 1.6245 |
| <partinfo>BBa_K300088</partinfo> | 74.8806 ± 2.7699 |
| <partinfo>BBa_K300090</partinfo> | 75.9433 ± 3.6808 |
| <partinfo>BBa_K300099</partinfo> | 78.4634 ± 2.5622 |
| <partinfo>BBa_B0031</partinfo> | 70.8421 ± 2.2181 |
Discussion
All cell cultures showed a similar growth curve; doubling time was computed as described here in order to have informations about the burden due to the synthesis of such fusion proteins. It's possible to see that all doubling time are very similar; it's possible to assert that the expression of these BioBrick parts doesn't cause abnormal stress to the cells.
From GFP curve it's possible to appreciate that in <partinfo>BBa_K300086</partinfo>, <partinfo>BBa_K300088</partinfo>, <partinfo>BBa_K300090</partinfo>, <partinfo>BBa_K300099</partinfo> GFP accumulation it's very similar and it's significantly different from that of negative control <partinfo>BBa_B0031</partinfo>. These results show that the green fluorescent protein assembled downstream of the genetic circuit is correctly folded.
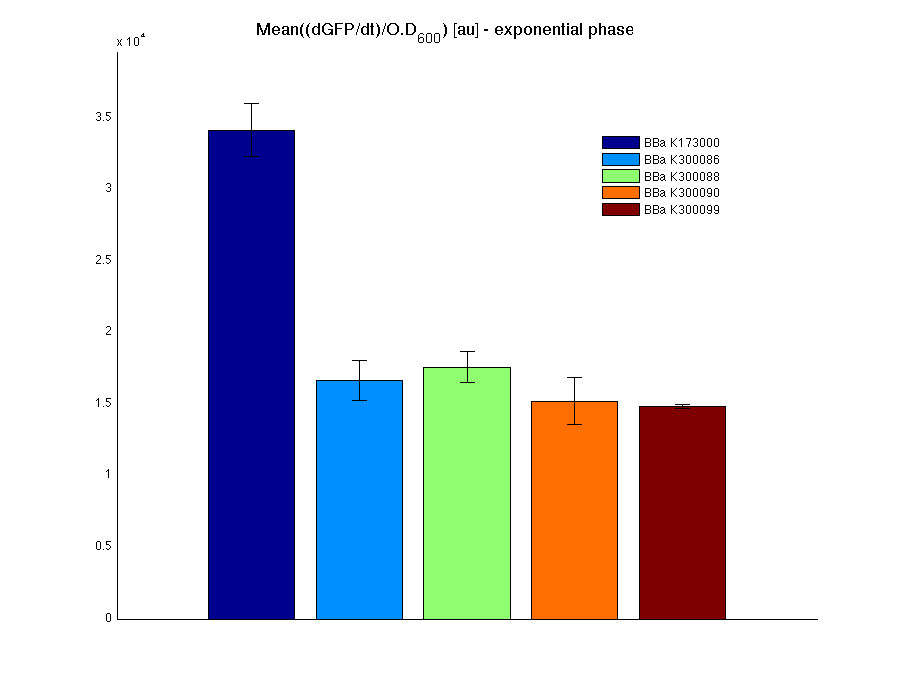
The mean protein synthesis rate was also computed over the growth exponential phase, showing again an appreciable GFP production rate that is about a half of the positive control.
3OC6HSL inducible devices
Methods
Inoculum (into 5 ml LB+Amp) from glycerol stock of:
- <partinfo>BBa_K300091</partinfo>
- <partinfo>BBa_K300092</partinfo>
- <partinfo>BBa_K173000</partinfo> (positive control)
- <partinfo>BBa_B0031</partinfo> (negative control)
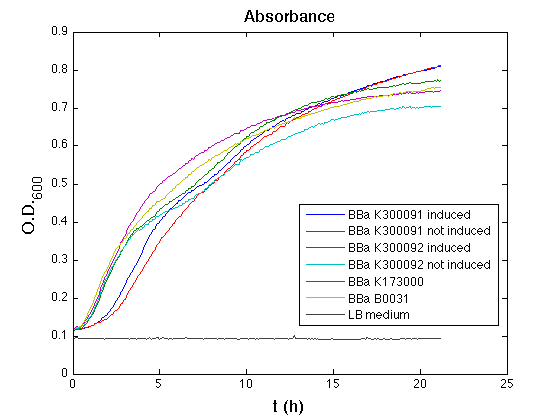
Cultures were grown ON at 37°C, 220 rpm.
The following day cultures were diluted 1:100 and let grow again for about five hours at 37°C, 220 rpm.
The optical density (O.D.) of each cell culture was than measured with TECAN Infinte F200. Samples were diluted in order to obtain the same O.D. equal to 0,02.
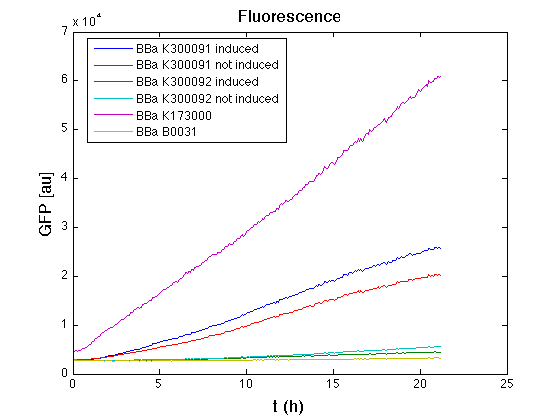
Then we performed a 21-hours' experiment with measurements of absorbance and green fluorescence every five minutes using TECAN Infinite F200; cultures were shaken for 15 seconds every five minutes. <partinfo>BBa_K300091</partinfo> and <partinfo>BBa_K300092</partinfo> circuits were induced 100nM with HSL directly into multiplate well. Each value shown below is the mean of three measurements, from GFP data that of a non-fluorescent culture (negative control) was subtracted.
Results
| Culture | Doubling time [min.] ± std error |
|---|---|
| <partinfo>BBa_K173000</partinfo> | 76.3336 ± 1.4362 |
| <partinfo>BBa_K300091</partinfo> induced | 121.1434 ± 7.0275 |
| <partinfo>BBa_K300091</partinfo> not induced | 74.4267 ± 1.3696 |
| <partinfo>BBa_K300092</partinfo> induced | 122.6088 ± 1.2785 |
| <partinfo>BBa_K300092</partinfo> not induced | 71.5105 ± 2.7113 |
| <partinfo>BBa_B0031</partinfo> | 70.8421 ± 2.2181 |
Discussion
All cell cultures showed a similar growth curve; doubling time was computed as described here in order to have informations about the burden due to the synthesis of such fusion proteins. It's possible to see that all doubling time are very similar except for induced cultures. In this case doubling time is much higher than posite control and not induced cultures; so it's possible to assert that in this case there's a kind of metabolic burden higher than in the others, maybe because of the inducible system.
From GFP curve it's possible to appreciate that in induced <partinfo>BBa_K300091</partinfo> and <partinfo>BBa_K300092</partinfo> GFP accumulation profile it's very similar and it's significantly different from that of negative control <partinfo>BBa_B0031</partinfo>. On the other hand not induced <partinfo>BBa_K300091</partinfo> and <partinfo>BBa_K300092</partinfo> show a profile very similar to the last one. These results show that the green fluorescent protein assembled downstream of the genetic circuit is correctly folded and that the inducible system works as expected.
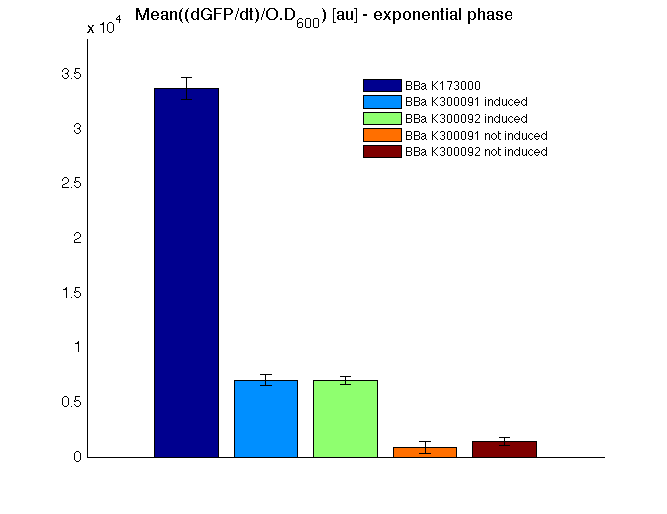
The mean protein synthesis rate was also computed over the growth exponential phase, showing again an GFP production rate that is different from negative control. Not induced <partinfo>BBa_K300091</partinfo> and <partinfo>BBa_K300092</partinfo> show a low GFP synthesis rate maybe due to 3OC6HSL inducible circuit leakage activity.
 "
"