Team:UT-Tokyo/Sudoku lab note
From 2010.igem.org
(→Assay I) |
(→Assay I) |
||
| Line 121: | Line 121: | ||
- Prepare 8 Erlenmeyer flask, pour 100mL LB broth, add 100 uL ampicillin, and 1 mL full growth medium. <br/> | - Prepare 8 Erlenmeyer flask, pour 100mL LB broth, add 100 uL ampicillin, and 1 mL full growth medium. <br/> | ||
- Make arabinose diluted solutions. <br/> | - Make arabinose diluted solutions. <br/> | ||
| - | + | 1. Add “L-(+)-Arabinose, minimum 99%” 1.5 g into 10ml arabinose to make master mix solution of 1.0x10^(-2)M arabinose for sample A.<br/> | |
| - | + | 2. Add 150 ul master mix solution to 1350 ul LB broth to make 1.0x10<sup>-3</sup>M arabinose solution for sample B.<br/> | |
3. Add 150 ul 1.0x10<sup>-3</sup>M arabinose solution to 1350 ul LB broth to make 1.0x10<sup>-4</sup> arabinose solution for sample C.<br/> | 3. Add 150 ul 1.0x10<sup>-3</sup>M arabinose solution to 1350 ul LB broth to make 1.0x10<sup>-4</sup> arabinose solution for sample C.<br/> | ||
4. Add 150 ul 1.0x10<sup>-4</sup>M arabinose solution to 1350 ul LB broth to make 1.0x10<sup>-5</sup> arabinose solution for sample D.<br/> | 4. Add 150 ul 1.0x10<sup>-4</sup>M arabinose solution to 1350 ul LB broth to make 1.0x10<sup>-5</sup> arabinose solution for sample D.<br/> | ||
| Line 129: | Line 129: | ||
-Put flasks into shaking incubator (25 degree C, 180 rpm) and begin incubation. | -Put flasks into shaking incubator (25 degree C, 180 rpm) and begin incubation. | ||
-Measure OD600 of each sample every one hour. | -Measure OD600 of each sample every one hour. | ||
| - | *Add arabinose solution of each concentration to the medium when the OD600 almost reached 2.0. | + | * Add arabinose solution of each concentration to the medium when the OD600 almost reached 2.0. |
-Extract 1ml medium from the flask and put into 1.5ml Eppendorf tube every time measuring OD. Put the tube into icebox as soon as possible. Centrifuge the tube and throw away supernatant to make E.coli pellet. Preserve pellet in -20 degrees C freezer. | -Extract 1ml medium from the flask and put into 1.5ml Eppendorf tube every time measuring OD. Put the tube into icebox as soon as possible. Centrifuge the tube and throw away supernatant to make E.coli pellet. Preserve pellet in -20 degrees C freezer. | ||
| Line 136: | Line 136: | ||
Measure Fluorescence<br/> | Measure Fluorescence<br/> | ||
| - | + | ~~1. Add 100 ul 8M urea buffer into each pellet and make suspension solution. | |
| - | + | 2. Put the pellet in room temperature for 30 minutes. | |
3. Use sonicator to smash the cell. (10 seconds, 10% power.) | 3. Use sonicator to smash the cell. (10 seconds, 10% power.) | ||
4. Put the tube into icebox and cool it down. | 4. Put the tube into icebox and cool it down. | ||
Revision as of 04:51, 27 October 2010


Sudoku
Introduction System Lab note [ June / July / August / September - October ] Result Reference
Lab note
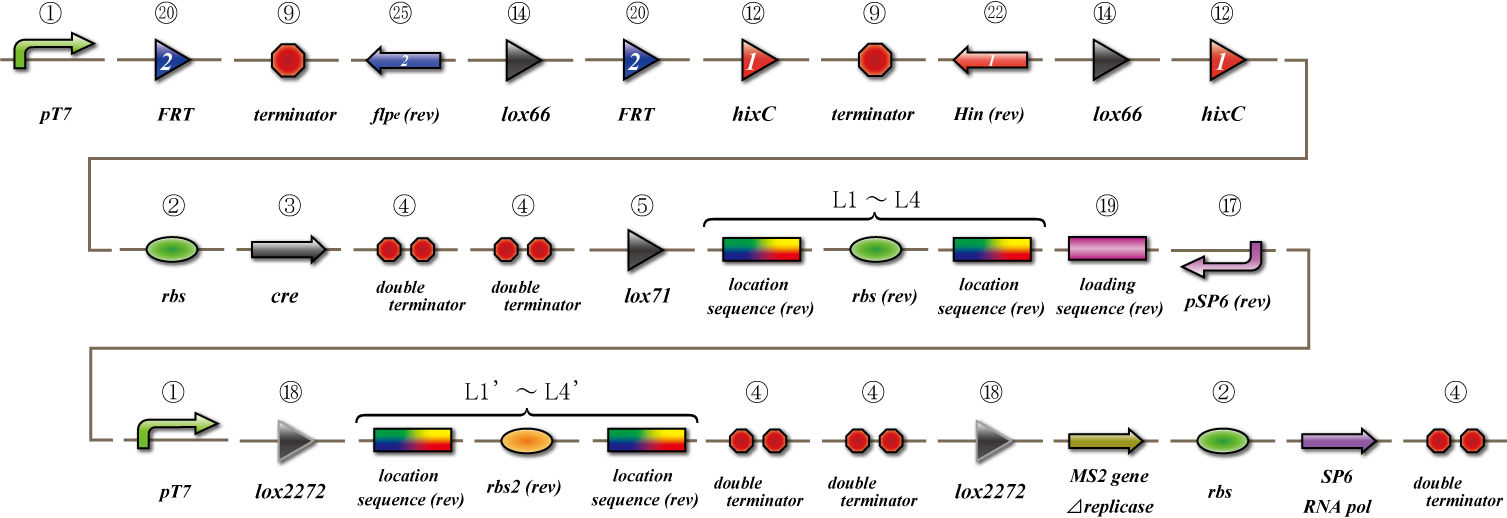
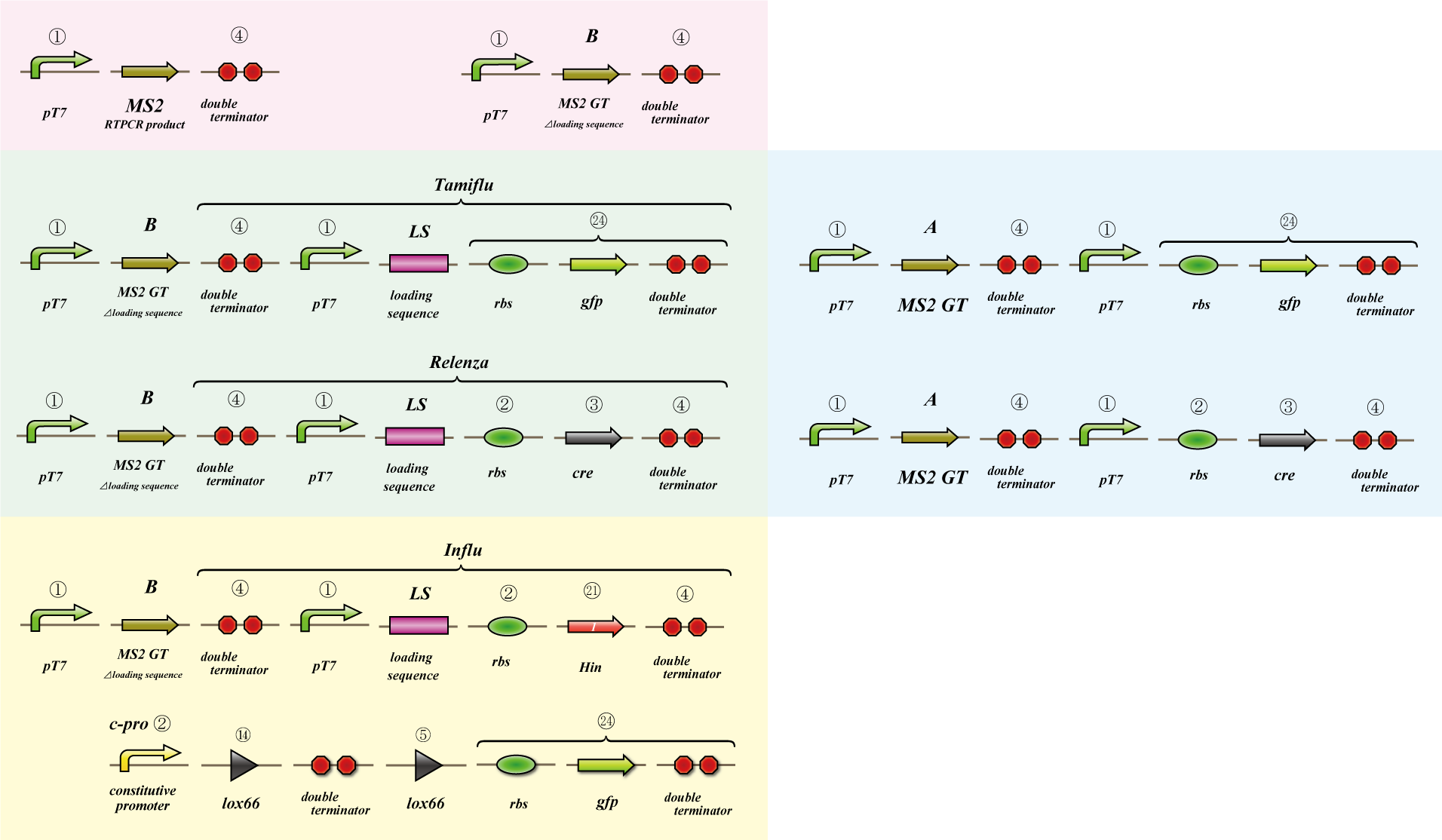
To make main construct, we made several assays.
3. #MS2 virus
4. #flpe
5. #Hin
Main construct
If you want to know detail explanation, please read Construct section.
Assay
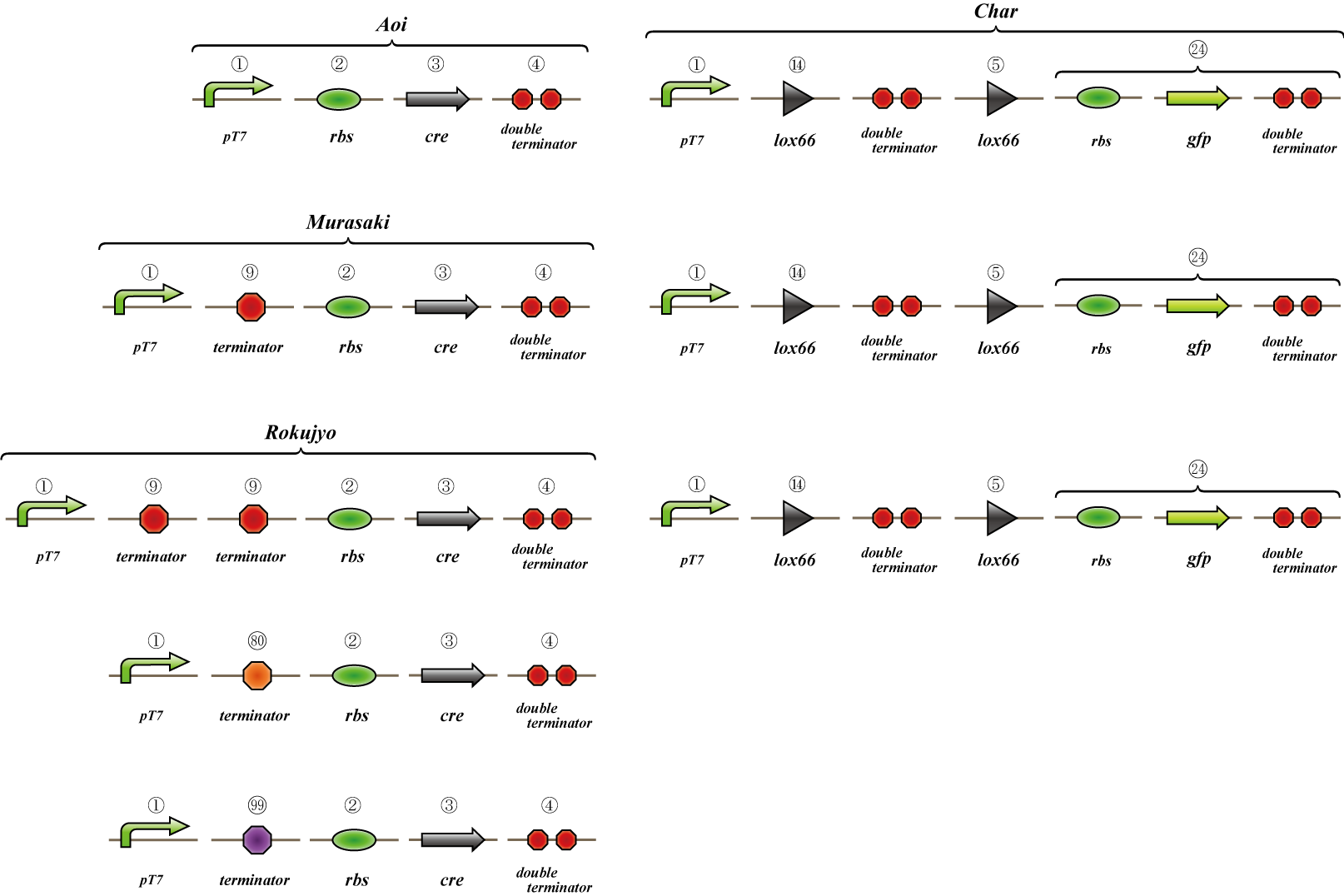
Terminator leak switch
To realize 4C3 leak switch, we should find a proper terminator which terminates transcription when connected two or more but leaks when single.
We made "A-M-Ro/Char" assay (see Fig, named from the famous animation, "MOBILE SUIT GUNDAM") to select proper terminator:
Aoi
no terminator
-> cre protein express rapidly
-> lox site is removed rapidly
-> gfp may expression rapidly
Murasaki
one terminator
-> cre protein express slowly
-> lox site is removed slowly
-> gfp may expression slowly
Rokujo
two terminators
-> cre protein can't express
-> lox site remain
-> gfp expression may not express
First we use single terminator BBa_B1006.
Then other terminators are tested: 80%-terminate terminator and 99%-terminate terminator to determine the best threshold to realize terminator leak switch.
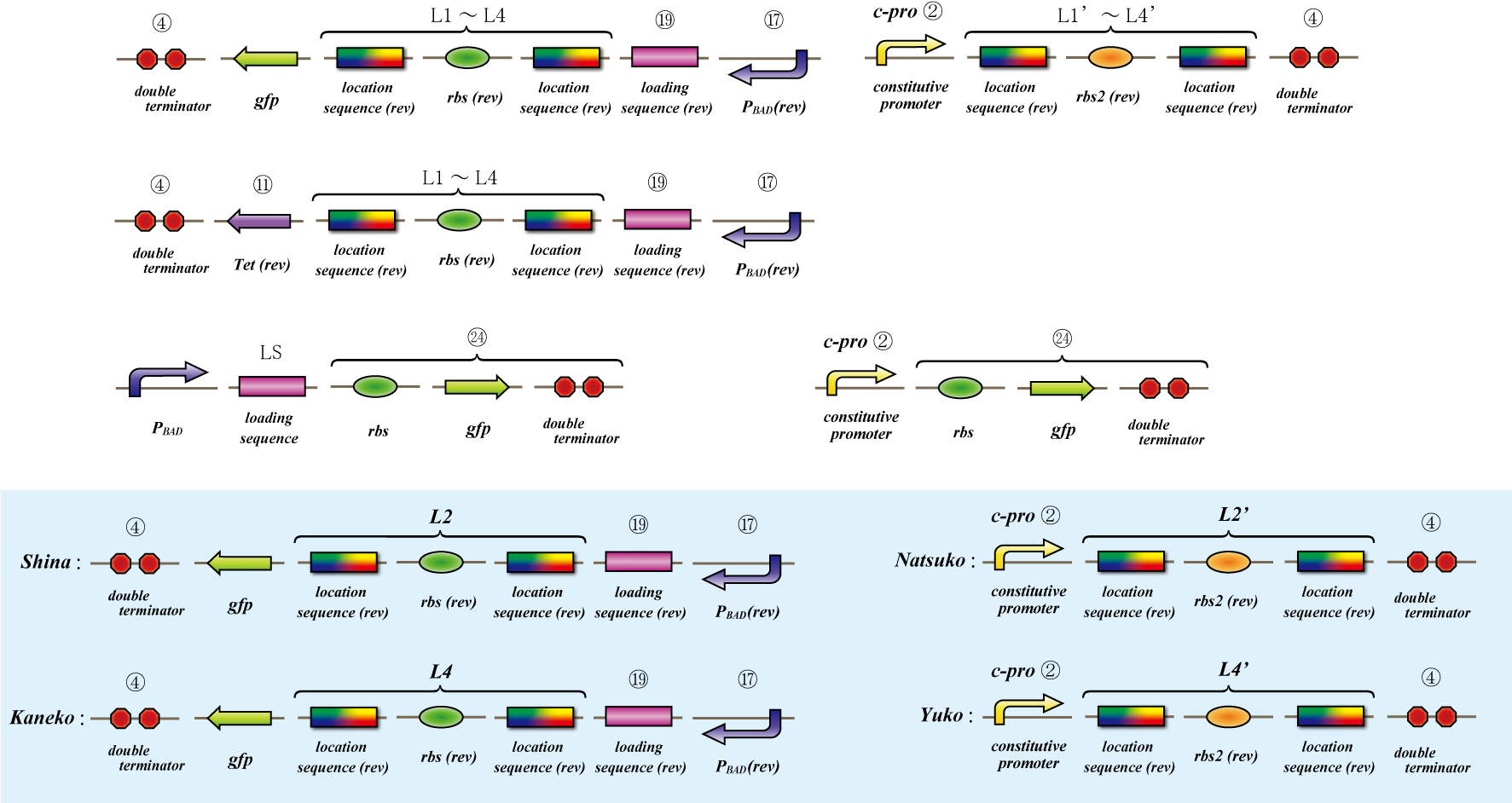
Location sequence
Location sequence assay protocol
Introduction
The object of the assay is to test whether antisense RNA used in our construct works or not. In order to block the unnecessary information transformed by the virus from the other grid, we use antisense RNA to block ribosome to bind the region around the ribosome binding site (rbs) and prevent the expression of protein. In our construct, information is carried by virus and antisense RNA is transcribed constantly inside the cell. Once the unnecessary information was transformed, the antisense will come and shut out all the RNA chain excluded by virus. In this assay, we used pBAD as the promoter to start translating grid information which will be transformed by virus in our construct. The strength of the promoter depends on the concentration of arabinose. On the other hand, we used c-pro as the promoter to start translating antisense RNA. This c-pro is the strongest constitutive promoter submitted in igem parts. In assay I, we examined the relative strength of pBAD (with eight different concentration of arabinose) and c-pro. By using the proper concentration of arabinose which was determined by assay I, in assay II, we inspect observe whether our antisense RNA works or not. In these two assay, gfp was used as a reporter protein.
Assay I
The assay to observe how strong pBAD expresses depending on concentration of arabinose.
1st day
Transform the miniprep product of pBAD-LS-24, pBAD-4, cpro-24, cpro-4. Incubate those plates at 37 degrees C for about twelve hours (until the diameter of the colony grows to nearly 1 cm).
2nd day
Make full growth medium for eight samples (A to H).
- Sample A; pBAD-LS-24, add 1.0x10-2 M arabinose solution.
- Sample B; pBAD-LS-24, add 1.0x10-3 M arabinose solution.
- Sample C; pBAD-LS-24, add 1.0x10-4 M arabinose solution.
- Sample D; pBAD-LS-24, add 1.0x10-5 M arabinose solution.
- Sample E; pBAD-LS-24, add 1.0x10-6 M arabinose solution.
- Sample F; pBAD-LS-4
- Sample G; cpro-24
- Sample H; cpro-4
Pour 15 mL of LB-10 medium (with 100 uL ampicillin) into Erlenmeyer flask, add tip which picked up one colony from the plate of each sample.
Incubate flask at 37 degree C with shaking at 180 rpm for more than 12 hours (until the medium become the state of full growth).
3rd day
Measure Optical Density (600).
- Prepare 8 Erlenmeyer flask, pour 100mL LB broth, add 100 uL ampicillin, and 1 mL full growth medium.
- Make arabinose diluted solutions.
1. Add “L-(+)-Arabinose, minimum 99%” 1.5 g into 10ml arabinose to make master mix solution of 1.0x10^(-2)M arabinose for sample A.
2. Add 150 ul master mix solution to 1350 ul LB broth to make 1.0x10-3M arabinose solution for sample B.
3. Add 150 ul 1.0x10-3M arabinose solution to 1350 ul LB broth to make 1.0x10-4 arabinose solution for sample C.
4. Add 150 ul 1.0x10-4M arabinose solution to 1350 ul LB broth to make 1.0x10-5 arabinose solution for sample D.
5. Add 150 ul 1.0x10-5M arabinose solution to 1350 ul LB broth to make 1.0x10-6 arabinose solution for sample E.
-Put flasks into shaking incubator (25 degree C, 180 rpm) and begin incubation. -Measure OD600 of each sample every one hour.
- Add arabinose solution of each concentration to the medium when the OD600 almost reached 2.0.
-Extract 1ml medium from the flask and put into 1.5ml Eppendorf tube every time measuring OD. Put the tube into icebox as soon as possible. Centrifuge the tube and throw away supernatant to make E.coli pellet. Preserve pellet in -20 degrees C freezer.
4th day
Measure Fluorescence
~~1. Add 100 ul 8M urea buffer into each pellet and make suspension solution.
2. Put the pellet in room temperature for 30 minutes.
3. Use sonicator to smash the cell. (10 seconds, 10% power.)
4. Put the tube into icebox and cool it down.
5. Do 3 and 4 again.
6. Centrifuge the tube. 150rpm, 2 minutes, 4 degrees C.
7. Measure the fluorescence.
Assay II
Test whether location sequence works or not.
1st day Transform the miniprep product of 4 samples bellow. -4-gfp-L2-pBAD(rev)-cpro-L2’-4 -4-gfp-L2-pBAD(rev)-cpro-L4’-4 -4-gfp-L4-pBAD(rev)-cpro-L2’-4 -4-gfg-L4-pBAD(rev)-cpro-L4’-4 Incubate those plates at 37 degrees C for about twelve hours (until the diameter of the colony grows to nearly 1 cm).
2nd day
Make full growth medium for eight samples (A to H).
Sample A; 4-gfp-L2-pBAD(rev)-cpro-L2’-4, add 1.0x10^(-2) M arabinose solution.
Sample B; 4-gfp-L2-pBAD(rev)-cpro-L4’-4, add 1.0x10^(-2) M arabinose solution.
Sample C; 4-gfp-L4-pBAD(rev)-cpro-L2’-4, add 1.0x10^(-2) M arabinose solution.
Sample D; 4-gfg-L4-pBAD(rev)-cpro-L4’-4, add 1.0x10^(-2) M arabinose solution.
Sample E; 4-gfp-L2-pBAD(rev)-cpro-L2’-4, add 1.0x10^(-4) M arabinose solution.
Sample F; 4-gfp-L2-pBAD(rev)-cpro-L4’-4, add 1.0x10^(-4) M arabinose solution.
Sample G; 4-gfp-L4-pBAD(rev)-cpro-L2’-4, add 1.0x10^(-4) M arabinose solution.
Sample H; 4-gfg-L4-pBAD(rev)-cpro-L4’-4, add 1.0x10^(-4) M arabinose solution.
Pour 15 mL of LB-10 medium (with 100 uL ampicillin) into Erlenmeyer flask, add tip which picked up one colony from the plate of each sample.
Incubate flask at 37 degree C with shaking at 180 rpm for more than 12 hours (until the medium become the state of full growth).
3rd day
Measure Optical Density (600).
-Prepare 8 Erlenmeyer flask, pour 100mL LB broth, add 100 uL ampicillin, and 1 mL full growth medium. -Put flasks into shaking incubator (25 degree C, 180 rpm) and begin incubation. -Measure OD600 of each sample every one hour.
- Add arabinose solution of each concentration to the medium when the OD600 almost reached 2.0.
-Extract 1ml medium from the flask and put into 1.5ml Eppendorf tube every time measuring OD. Put the tube into icebox as soon as possible. Centrifuge the tube and throw away supernatant to make E.coli pellet. Preserve pellet in -20 degrees C freezer.
4th day Measure Fluorescence
1. Add 100 ul 8M urea buffer into each pellet and make suspension solution. 2. Put the pellet in room temperature for 30 minutes. 3. Use sonicator to smash the cell. (10 seconds, 10% power.) 4. Put the tube into icebox and cool it down. 5. Do 3 and 4 again. 6. Centrifuge the tube. 150rpm, 2 minutes, 4 degrees C. 7. Measure the fluorescence.
Parts making
Ligate small parts - recognation site, location site and rbs.
Expression check
This assay testifies whether translation repression by location sequence go well.
First we check the ability of pBAD, which is induced by arabinose, by the expression of tetracycline-tolerance protein.
Then to check translation repression, we make assay which express gfp only when translation repression can’t be occurring.
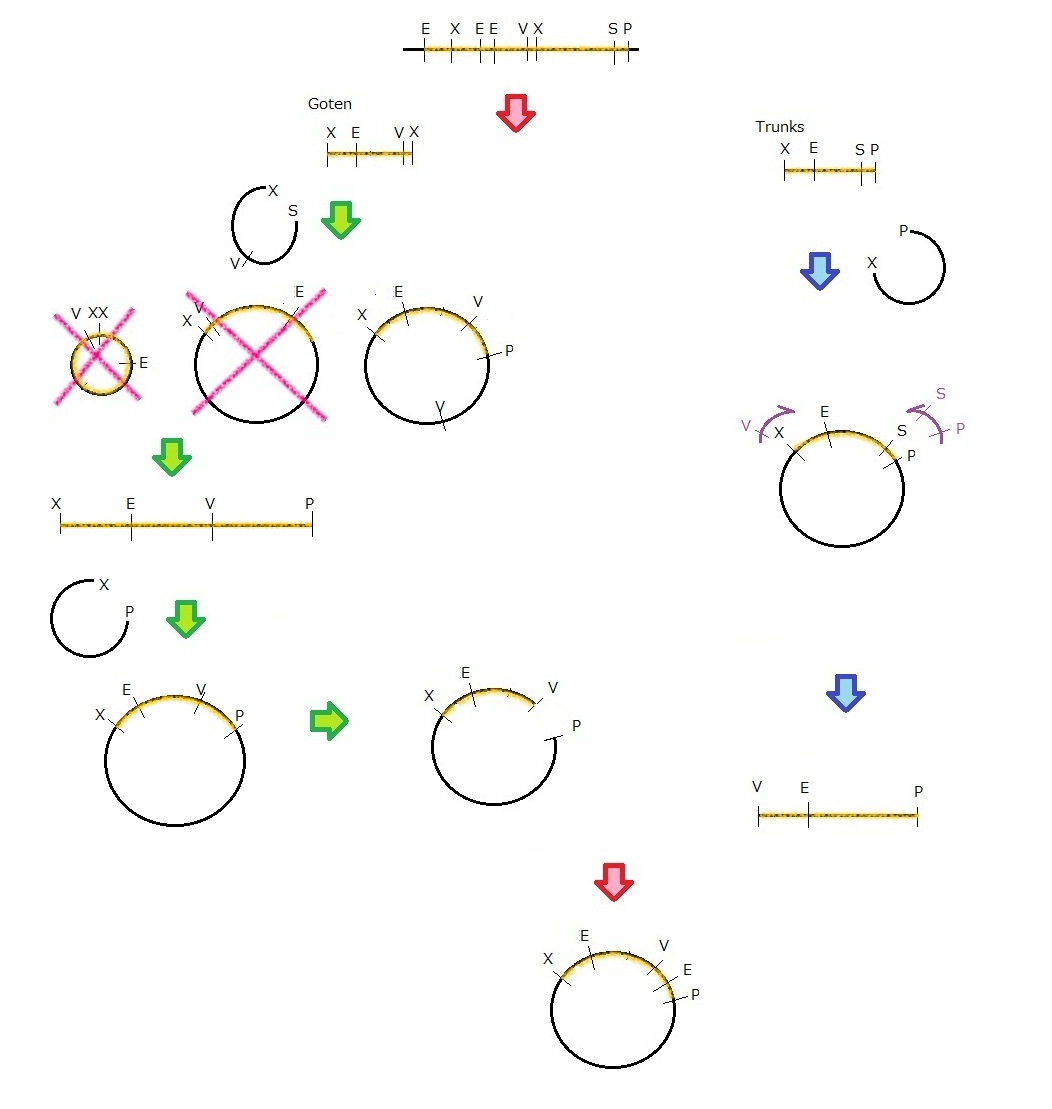
MS2 virus
Parts making
We get MS2 gene RT-PCR product. This original product include a lot of restricted enzyme site: two EcoRI site, two XbaI site and one VspI site.
To run our project, we don’t have to remove EcoRI site in the region. So we modified XbaI site by PCR as the following method:
1. We divided RT-PCR product into two parts by XP enzyme digestion:
E-X-E-E-V-X -> “Goten”
X-S-P -> “Tranks”
(named from the famous animation, "DRAGON BALL")
2. Insert is ligated with the vector, chloramphenicol-tolerance:
Goten -> EX vector
Tranks -> XP vector
3. Tranks -> PCR adding V-X region
4. Each part -> VP enzyme digestion, ligation each other
Expression check
We use MS2 phage to transmit information of location and number. The expression of the phage should start after 4C3 leak switch turns on. MS2 phage transport RNA which has loding sequence, so we knock out self assembly and made our E.coli translate loading seaquence at another point. To check whether transportion go correctly, we made assay.
i. Phage expression assay (red region)
Check whether RT-PCR product can be translated into MS2 phage.
ii. Packaging assay (blue & green region)
MS2 phage package RNA which has loading sequence. Using this character, we check whether RNA which has loading sequence and other coding region (gfp, cre) can be packaged correctly.
iii. Infection assay (yellow & green region)
We make the assay, which express gfp only when cre protein is expressed correctly. By using this part, we check whether E.coli can be infected with the MS2 phage made in assay ii as the fluorescence of gfp.
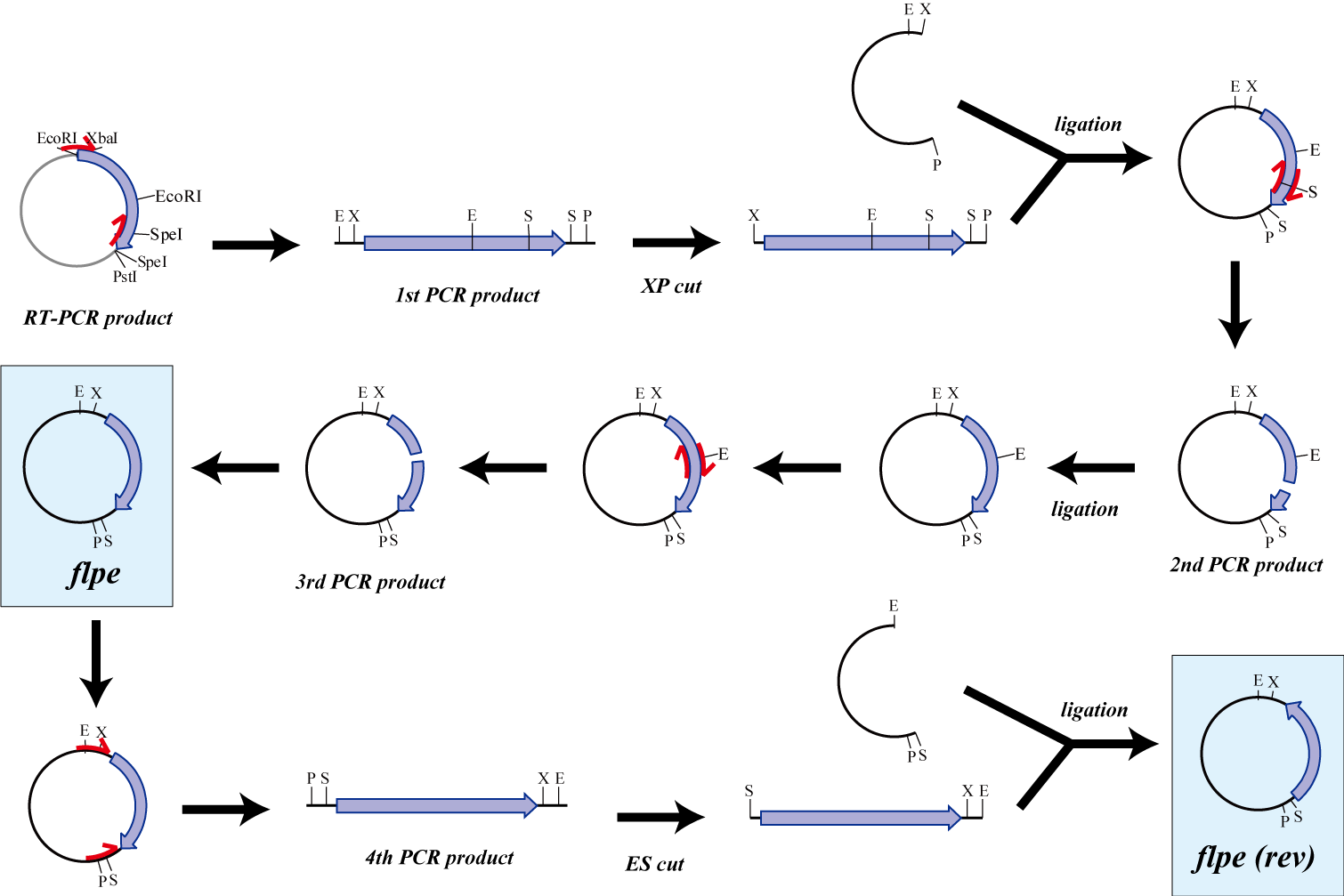
flpe
Parts making
The original flpe include two restriction enzyme sites(EcoRI, SpeI).
We modified this flpe by PCR:
1st PCR : cloning
-> ligation with vector
-> 2nd PCR : modified SpeI site
-> 3rd PCR : modified EcoRI site
We use this part as the form of reverse, so we made this part reverse by PCR.
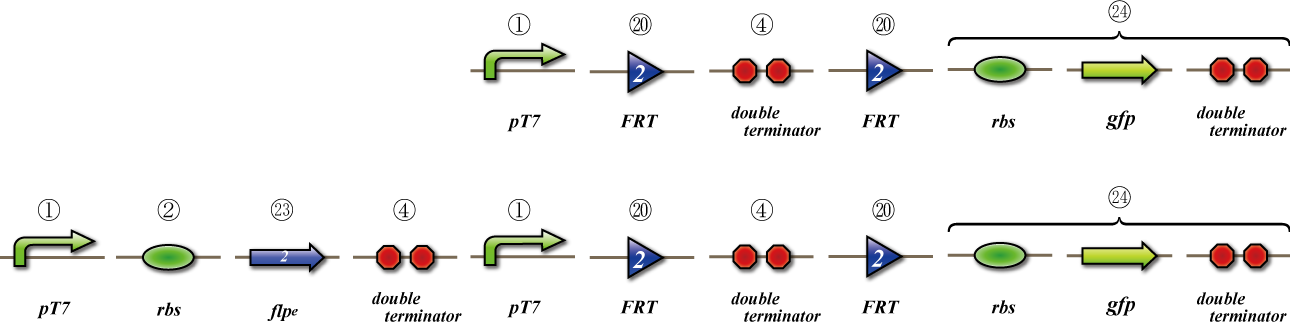
Expression check
To check whether flpe works correctly, we made assay shown in Fig.
The top construct is a nagative control. GFP can't be expressed because of the double terminators.
The bottom construct express GFP when flpe works correctly. When flpe is expressed correctly, flpe recognaize the frt site and double terminator which terminates the expression of gfp is removed, so GFP may be expressed.
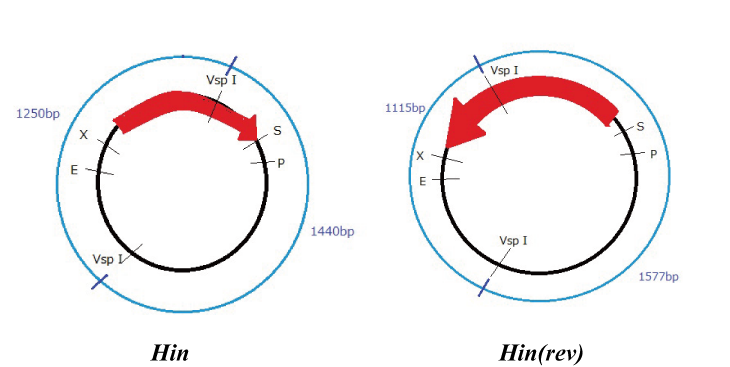
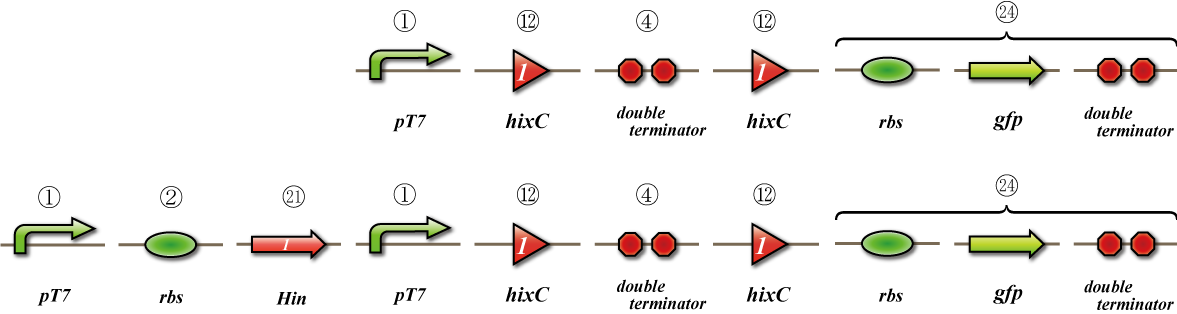
Hin
Parts making (reverse)
We get Hin parts from HQ (BBa_J31000).
We use this part as the form of reverse, so we made this part reverse by PCR.
To check PCR is done correctly, we did VspI digestion.
Expression check
To check whether flpe works correctly, we made assay shown in Fig, similar to the assay of flpe check.
The top construct is a nagative control. GFP can't be expressed because of the double terminators.
The bottom construct express GFP when Hin works correctly. When Hin is expressed correctly, Hin recognaize the hix site and double terminator which terminates the expression of gfp is removed, so GFP may be expressed.
Parts list
| Number | Name | Link to BioBrick | Plate coordinate | Vector | Code length |
| 1 | T7 promoter | plate1-6N | pSB1AK8 | 46bp | |
| 2 | rbs | plate1-1H | pSB1A2 | 15bp | |
| 3 | cre recombinase | plate1-5D | pSB1A2 | 1037bp | |
| 4 | double terminator | plate2-24C | pSB1AK3 | 95bp | |
| 5 | lox66 recombinase site | plate1-17J | pSB1A2 | 34bp | |
| 6 | Kan resistance (rev) | plate1-2K | pSB1A2 | 816bp | |
| 7 | rbs (rev) | plate1-1J | pSB1A2 | 15bp | |
| 8 | lox71 recombinase site |
order-made | --- | --- | 34bp |
| 9 | single terminator | plate1-4H | pSB1AK3 | 39bp | |
| 10 | constant express promoter | plate1-18A | pSB1A2 | 35bp | |
| 11 | Tet resistance (rev) | plate1-1N | pSB1A2 | 1191bp | |
| 12 | hixC | plate1-1B | pSB1A2 | 26bp | |
| 13 | --- |
--- | --- | --- | --- |
| 14 | lox66 | plate1-17H | pSB1A2 | 34bp | |
| 15 | rbs-mRFP1-terminator | plate1-22O | pSB1A2 | 861bp | |
| 16 | location sequence(old) |
order | --- | --- | --- |
| 17 | pSP6(rev) |
order | --- | --- | --- |
| 18 | lox2272 |
order | --- | --- | --- |
| 19 | loading sequence(rev) |
order | --- | --- | --- |
| 20 | frt | From HQ | pSB1A2 | 34bp | |
| 21 | hin(true) | From HQ | ??? | 573bp | |
| 22 | hin(rev) |
--- | --- | ??? | 573bp |
| 23 | flpe |
--- | --- | --- | about 1.2kbp |
| 24 | gfp unit |
--- | --- | --- | about 1.2kbp |
| 25 | flpe(rev) |
--- | --- | --- | about 1.2kbp |
Detail protocols
Detail protocols about this is:
June.
July.
 "
"