Team:TU Delft/19 July 2010 content
From 2010.igem.org
| Line 1: | Line 1: | ||
==Lab work== | ==Lab work== | ||
| + | |||
| + | <h4>Ordered DNA + Solvent Tolerance and Hydrocarbon Sensing</h4> | ||
| + | The frozen pellet of [https://2010.igem.org/Team:TU_Delft/16_July_2010_content last week] were isolated using [[Team:TU_Delft/protocols/birnboim_plasmid_isolation|Birnboim protocol]]. We used 3 mL of the bacterial cells to make [[Team:TU_Delft/protocols/freezing_bacterial_stocks|-80 °C stocks]]. | ||
| + | The following plasmid concentrations were obtained: | ||
| + | {| style="color:black; background-color:white;" cellpadding="5" cellspacing="0" border="1" | ||
| + | |'''BioBrick''' | ||
| + | |'''Composed of''' | ||
| + | |'''Concentration (ng/μL)''' | ||
| + | |- | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |- | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |- | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |- | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |- | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |- | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |- | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |} | ||
| + | |||
| + | Because not all [https://2010.igem.org/Team:TU_Delft/14_July_2010_content ligation mixes] resulted in transformants were repeated the [[Team:TU_Delft/protocols/transformation|transformation]] with the overnight ligated reactions. | ||
<h4>Alkane degradation</h4> | <h4>Alkane degradation</h4> | ||
Revision as of 20:30, 19 July 2010
Contents |
Lab work
Ordered DNA + Solvent Tolerance and Hydrocarbon Sensing
The frozen pellet of last week were isolated using Birnboim protocol. We used 3 mL of the bacterial cells to make -80 °C stocks. The following plasmid concentrations were obtained:
| BioBrick | Composed of | Concentration (ng/μL) |
Because not all ligation mixes resulted in transformants were repeated the transformation with the overnight ligated reactions.
Alkane degradation
Biobricks in production:
| # | Digestion reaction | Used Buffer | Needed fragment |
| 1 | 2 μg J61100 + EcoRI + SpeI | Buffer 2 (BioLabs) | ‘E – J61100 – S’ |
| 2 | 2 μg J61100 + EcoRI + SpeI | Buffer 2 (BioLabs) | ‘E – J61100 – S’ |
| 3 | 1 μg rubR + EcoRI + SpeI | Buffer 2 (BioLabs) | ‘E – rubR– S’ |
| 4 | 1 μg J61101 + EcoRI + SpeI | Buffer 2 (BioLabs) | ‘E – J61101– S’ |
| 5 | 1 μg J61107 + EcoRI + SpeI | Buffer 2 (BioLabs) | ‘E – J61107 – S’ |
| 6 | 1 μg alkB2 + Xbal + PstI | Buffer 2 (BioLabs) | ‘X – alkB2 – P’ |
| 7 | 1 μg rubA3 + Xbal + PstI | Buffer 2 (BioLabs) | ‘X – rubA3 – P’ |
| 8 | 1 μg rubA4 + Xbal + PstI | Buffer 2 (BioLabs) | ‘X – rubA4 – P’ |
| 9 | 1 μg B0015 + Xbal + PstI | Buffer 2 (BioLabs) | ‘X – B0015 – P’ |
| 10 | 1 μg ladA + Xbal + PstI | Buffer 2 (BioLabs) | ‘X – ladA – P’ |
| 11 | 1 μg ADH + Xbal + PstI | Buffer 2 (BioLabs) | ‘X – ADH – P’ |
| 12 | 1 μg ALDH + Xbal + PstI | Buffer 2 (BioLabs) | ‘X – ALDH – P’ |
| 13 | 3 μg pSB1T3 + EcoRI + PstI | Buffer 2 (BioLabs) | ‘E – ---- – P’ |
Emulsifier
Since last weeks attempt to construct the inducible protomer R0011 with RBS B0032 failed, we decided to take a different approach. Instead of doing the standard 2 parts assembly, we amplify the promoter by PCR digest it and ligate in the plasmid that contains the RBS which has only be cut open at the left side. By doing so we do not risk losing the super small DNA fragments like the RBS. The problem is that we cannot use antibiotics selection. So we need to determine that by colony PCR and sequencing.
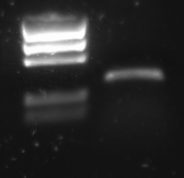
PCR Amplification
R0011 was amplified with the universal primers G00100 and G00101. The product was put on 1% agarose gel:
Lane description:
| # | Description |
| 1 | BioRad EZ Ladder (5 μL) |
| 2 | PCR Product of R0011 (10 μL + 2 μL Loading buffer) |
The PCR band is about 300 bps long. R0011 itself is just 55 bp, but the flanking primer regions are about 100 bp each.
Digestion
The PCR product of promotor R0011 was cut with EcoRI and SpeI and the RBS containing plasmid has been cut open with EcoRI and Xbal for 2.5 hours at 37 °C. This deviates from the standard biobrick assembly, thus is not completely as described in our digestion protocol.
| # | Digestion reaction | Used Buffer | Needed fragment |
| 1 | 1 μg B0032 + EcoRI + Xbal: | Buffer 2 (BioLabs) | ‘X – B0032 - Plasmid backbone – E’ |
| 2 | 30 μL PCR product of R0011 + EcoRI + SpeI | Buffer 2 (BioLabs) | ‘E - R0011 - S’ |
Ligation
The digestion products were ligated over night:
| # | Ligation reaction |
| 1 | 5 μL B0032 + 10 μL PCR Product of R0011 |
Hopefully this will lead to 'E - X - R0011 - B0032 - S - P' in the B0032 plasmid backbone with Ampicillin resistance marker.
 "
"