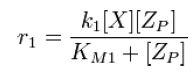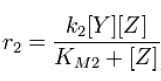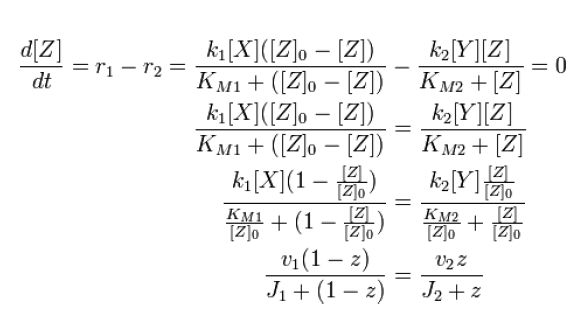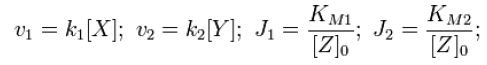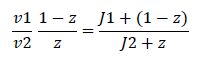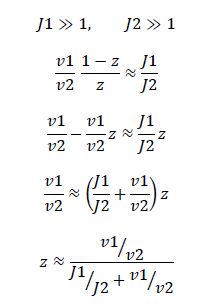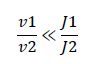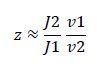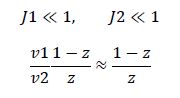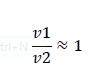Team:Stanford/Research/Modeling
From 2010.igem.org
Drummstikk (Talk | contribs) (→Kinase/Phosphatase System) |
(→Kinase/Phosphatase System) |
||
| Line 7: | Line 7: | ||
==Kinase/Phosphatase System== | ==Kinase/Phosphatase System== | ||
Throughout this derivation, we refer to Goldbeter and Koshland's seminal work on the kinetics of emzyme pairs [[Team:Stanford/Research/Modeling#References| [1]]]. | Throughout this derivation, we refer to Goldbeter and Koshland's seminal work on the kinetics of emzyme pairs [[Team:Stanford/Research/Modeling#References| [1]]]. | ||
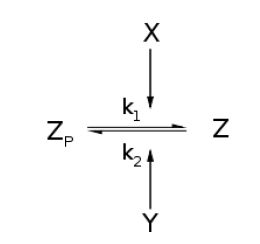
| - | For an opposing pair of enzymes X and Y modifying and unmodifying a substrate Z with modification p, | + | For an opposing pair of enzymes ''X'' and ''Y'' modifying and unmodifying a substrate ''Z'' with modification ''p'', |
[[Image:MainGBK.JPG|center]] | [[Image:MainGBK.JPG|center]] | ||
| - | From Michaelis-Menten kinetics we know that the rate at which Zp is dephosphorylated is | + | From Michaelis-Menten kinetics we know that the rate at which ''Zp'' is dephosphorylated is |
[[Image:R1.JPG]][[Team:Stanford/Research/Modeling#References| [2]]] | [[Image:R1.JPG]][[Team:Stanford/Research/Modeling#References| [2]]] | ||
| - | and the rate at which Z is phosphorylated is | + | and the rate at which ''Z'' is phosphorylated is |
[[Image:R2.JPG]][[Team:Stanford/Research/Modeling#References| [2]]] | [[Image:R2.JPG]][[Team:Stanford/Research/Modeling#References| [2]]] | ||
| - | We also know that Z can be modified an unmodified, but its total is conserved: [Z]o = [Zp] + [Z] | + | We also know that ''Z'' can be modified an unmodified, but its total is conserved: ''[Z]o = [Zp] + [Z]'' |
At steady state, modification and unmodification rates are equal, leading us to | At steady state, modification and unmodification rates are equal, leading us to | ||
| Line 61: | Line 61: | ||
[[Image:8thblock.JPG|center]] | [[Image:8thblock.JPG|center]] | ||
| - | When the enzymes are saturated, the only steady-state solution is v1 = v2. Any ratio of v1/v2 below this, and all the substrate becomes modified. Any ratio above and all the substrate becomes unmodified. Simply by changing the concentration of substrate, we have converted our analog ratio sensor to a digital ratio sensor. | + | When the enzymes are saturated, the only steady-state solution is'' v1'' = ''v2''. Any ratio of ''v1/v2'' below this, and all the substrate becomes modified. Any ratio above and all the substrate becomes unmodified. Simply by changing the concentration of substrate, we have converted our analog ratio sensor to a digital ratio sensor. |
The requirements for this digital ratio sensor are: | The requirements for this digital ratio sensor are: | ||
Revision as of 03:12, 28 October 2010

| Home | Project | Applications | Modeling | Parts | Team | Notebook |
Goals
Our intuition for what makes a good ratio sensor could only take us so far. From the very first stages of design, we wanted to back up and test our ideas with mathematical tools. Luckily, we found that solving the equations of mass action kinetics at steady-state was enough to give us clear design criteria. We present the mathematical basis for sensors that are capable of sensing a single ratio digitally, or many ratios in an analog fashion.
Kinase/Phosphatase System
Throughout this derivation, we refer to Goldbeter and Koshland's seminal work on the kinetics of emzyme pairs [1]. For an opposing pair of enzymes X and Y modifying and unmodifying a substrate Z with modification p,
From Michaelis-Menten kinetics we know that the rate at which Zp is dephosphorylated is
and the rate at which Z is phosphorylated is
We also know that Z can be modified an unmodified, but its total is conserved: [Z]o = [Zp] + [Z]
At steady state, modification and unmodification rates are equal, leading us to
where
Rearranging gives
Now, assume that the two opposing enzymes are nowhere near being saturated with substrate:
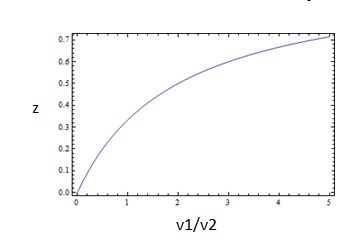
So, the fraction of modified substrate is a saturating function of the ratio of inputs. The half-max value is the ratio of the strengths of the two enzymes. The linear regime of this function exists where
,and the fraction of unmodified substrate is a linear function of the ratio of inputs,
,which is the equation for a Michaelis-Menten-shaped curve
We now know that the requirements for this analog ratio sensor are
- Consistent relationships between inputs and enzyme activities (non-trivial)
- A non-saturating amount of substrate
But what happens if we disobey requirement 1? Let's increase the amount of substrate to saturation so that
When the enzymes are saturated, the only steady-state solution is v1 = v2. Any ratio of v1/v2 below this, and all the substrate becomes modified. Any ratio above and all the substrate becomes unmodified. Simply by changing the concentration of substrate, we have converted our analog ratio sensor to a digital ratio sensor.
The requirements for this digital ratio sensor are:
- Consistent relationships between inputs and enzyme activities (non-trivial)
- An amount of substrate sufficient to saturate both opposing enzymes
References
[1] [http://www.ncbi.nlm.nih.gov/pubmed/6947258 Goldbeter A, Koshland DE Jr.: An amplified sensitivity arising from covalent modification in biological systems. PNAS. 1981 Nov;78(11)6840-4.]
[2] [http://en.wikipedia.org/wiki/Goldbeter-Koshland_kinetics Goldbeter-Koshland kinetics In Wikipedia. Retrieved July 13, 2010, from http://en.wikipedia.org/wiki/Goldbeter-Koshland_kinetics]
 "
"