Team:Newcastle/7 July 2010
From 2010.igem.org
Shethharsh08 (Talk | contribs) |
Shethharsh08 (Talk | contribs) |
||
| Line 40: | Line 40: | ||
==Results== | ==Results== | ||
* Bands were seen at the correct size for pMutin4 and pSB1AT3 and the amplified PCR product. | * Bands were seen at the correct size for pMutin4 and pSB1AT3 and the amplified PCR product. | ||
| + | |||
| + | N.B. To limit DNA exposure to UV radiation images were not taken using the geldoc and bands were extracted directly using UV transilluminator. | ||
==Conclusion== | ==Conclusion== | ||
| Line 46: | Line 48: | ||
==Inference== | ==Inference== | ||
* The purified PCR product is cut to give it sticky ends complementary to the digested pSB1AT3. pSB1AT3 is excised from the gel ready for gel extraction. | * The purified PCR product is cut to give it sticky ends complementary to the digested pSB1AT3. pSB1AT3 is excised from the gel ready for gel extraction. | ||
| + | |||
| + | {{Team:Newcastle/footer}} | ||
Revision as of 15:57, 25 October 2010

| |||||||||||||
| |||||||||||||
Contents |
Chromosomal prep
Aim
To test whether the chromosome extraction from Bacillus subtilis ATCC6633 was successful.
Materials and Procedure
Please refer to: PCR and Gel electrophoresis for materials and protocol. We used ara and sac forward and reverse primers for amplification.
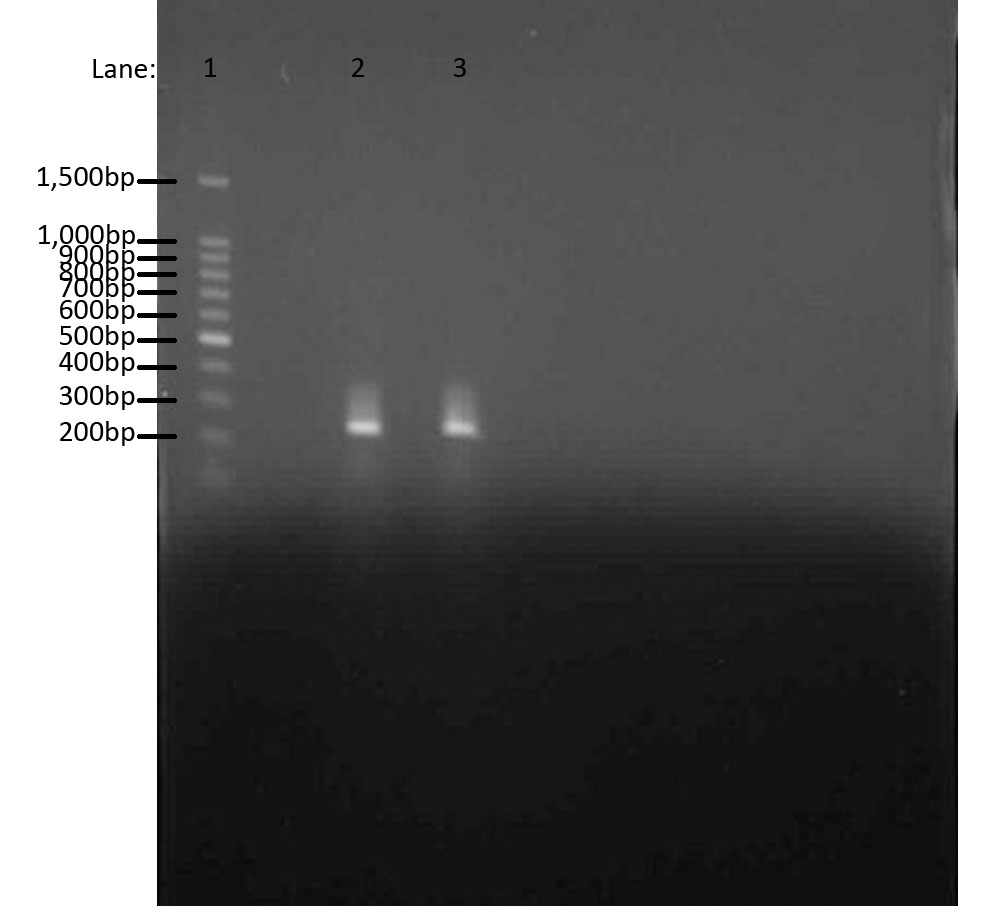
Results
We used Gel Electrophoresis to test whether we have the DNA we wanted. As we already know, the ara gene is about 300bps. We used 100 bp DNA ladder as a guidance for our bands. The two bands produced were from two separate ara cultures and they indeed show the bands in the same region, i.e. 300bps.
- Lane 1: 100 bp DNA ladder
- Lane 2: Chromosomal DNA with ara gene 1
- Lane 3: Chromosomal DNA with ara gene 2
Conclusion
The aim of this whole experiment was to extract genomic DNA from Bacillus subtilis ATCC 6633. In order to test whether we had extracted the DNA, we first used PCR to amplify and then used Gel Electrophoresis to compare the bands of ara genes to the 100 bp ladder. It worked!
LacI BioBrick Construction
Aims
- To use PCR to extract lacI (promoter, ribosome-binding site (RBS) & coding sequence (CDS)) from plasmid pMutin4 and ligate into vector pSB1AT3 in front of red fluorescent protein (RFP).
Materials
- Digested plasmids pMutin4 and pSB1AT3
- PCR product
- Restriction enzymes EcoR1 and Spe1
Protocol
- The digests and PCR product are run on an agarose gel
- pSB1AT3 is excised from the gel
- lacI PCR product is purified.
- Purified lacI is restriction digested with EcoR1 and Spe1.
Results
- Bands were seen at the correct size for pMutin4 and pSB1AT3 and the amplified PCR product.
N.B. To limit DNA exposure to UV radiation images were not taken using the geldoc and bands were extracted directly using UV transilluminator.
Conclusion
- The digested plasmids and the PCR product were run on an agarose gel in order to determine what was in these samples. The samples should have run at . The samples did in fact run in this manner, and so it can be said with confidence that our protocols have worked as they should.
Inference
- The purified PCR product is cut to give it sticky ends complementary to the digested pSB1AT3. pSB1AT3 is excised from the gel ready for gel extraction.
 
|
 "
"