Team:Newcastle/7 July 2010
From 2010.igem.org
(Difference between revisions)
Shethharsh08 (Talk | contribs) (→Chromosomal prep) |
Shethharsh08 (Talk | contribs) |
||
| Line 21: | Line 21: | ||
==Conclusion== | ==Conclusion== | ||
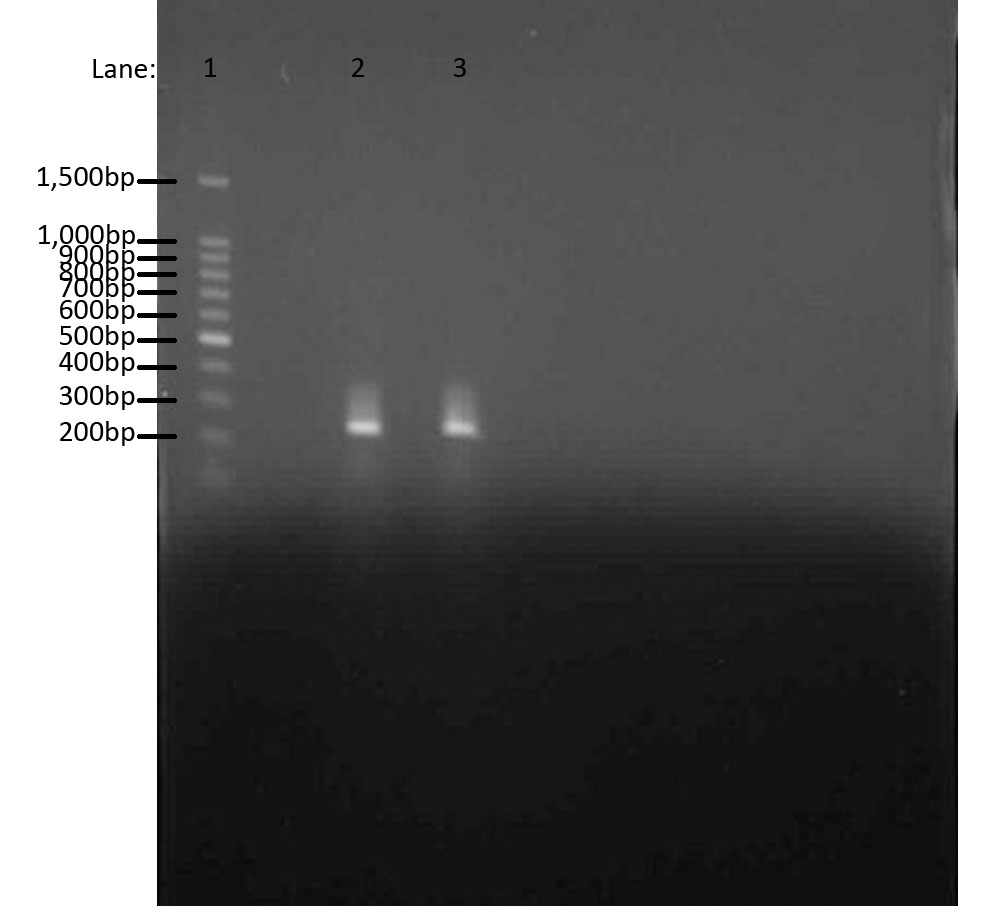
The aim of this whole experiment was to extract genomic DNA from ''B. subtilis'' strain ATCC 6633. In order to test whether we had extracted the DNA, we first used PCR to amplify and then used Gel Electrophoresis to compare the bands of ''ara'' genes to the 100 bp ladder. It worked! | The aim of this whole experiment was to extract genomic DNA from ''B. subtilis'' strain ATCC 6633. In order to test whether we had extracted the DNA, we first used PCR to amplify and then used Gel Electrophoresis to compare the bands of ''ara'' genes to the 100 bp ladder. It worked! | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Revision as of 14:37, 25 October 2010

| |||||||||||||
| |||||||||||||
Contents |
Chromosomal prep
Aim
To test whether the chromosome extraction from Bacillus subtilis ATCC6633 was successful.
Materials and Procedure
Please refer to: PCR and Gel electrophoresis for materials and protocol. We used ara and sac forward and reverse primers for amplification.
Results
We used Gel Electrophoresis to test whether we have the DNA we wanted. As we already know, the ara gene is about 300bps. We used 100 bp DNA ladder as a guidance for our bands. The two bands produced were from two separate ara cultures and they indeed show the bands in the same region, i.e. 300bps.
- Lane 1: 100 bp DNA ladder
- Lane 2: Chromosomal DNA with ara gene 1
- Lane 3: Chromosomal DNA with ara gene 2
 "
"