Team:Michigan/Protocols
From 2010.igem.org
(Difference between revisions)
(→Protocols) |
(→Oil Sands) |
||
| Line 64: | Line 64: | ||
[[Media:8-6-2010_Biofilm_Formation_Experiment.pdf|Revised biofilm assay protocol]] | [[Media:8-6-2010_Biofilm_Formation_Experiment.pdf|Revised biofilm assay protocol]] | ||
*[[Media:7-28-2010_Biofilm_Formation_Experiment.pdf|biofilm assay protocol]] | *[[Media:7-28-2010_Biofilm_Formation_Experiment.pdf|biofilm assay protocol]] | ||
| + | |||
| + | [[Media:Static+biofilm+quantification.pdf|Alex's biofilm assay protocol]] | ||
| + | |||
[[Media:7-31-2010_Flu_operon_primers.pdf|General primer design]] | [[Media:7-31-2010_Flu_operon_primers.pdf|General primer design]] | ||
Revision as of 20:37, 29 September 2010
Protocols
Using Lab Equipment
Obtaining Deionized Water in the ERB
Enigeering Research Building Autoclave
Epifluorescence Microscope Usage - H.H. Dow
Cell Culture
Making cultures from a -80C freezer stock
P. putida KT2440 antibotic resistance tolerance
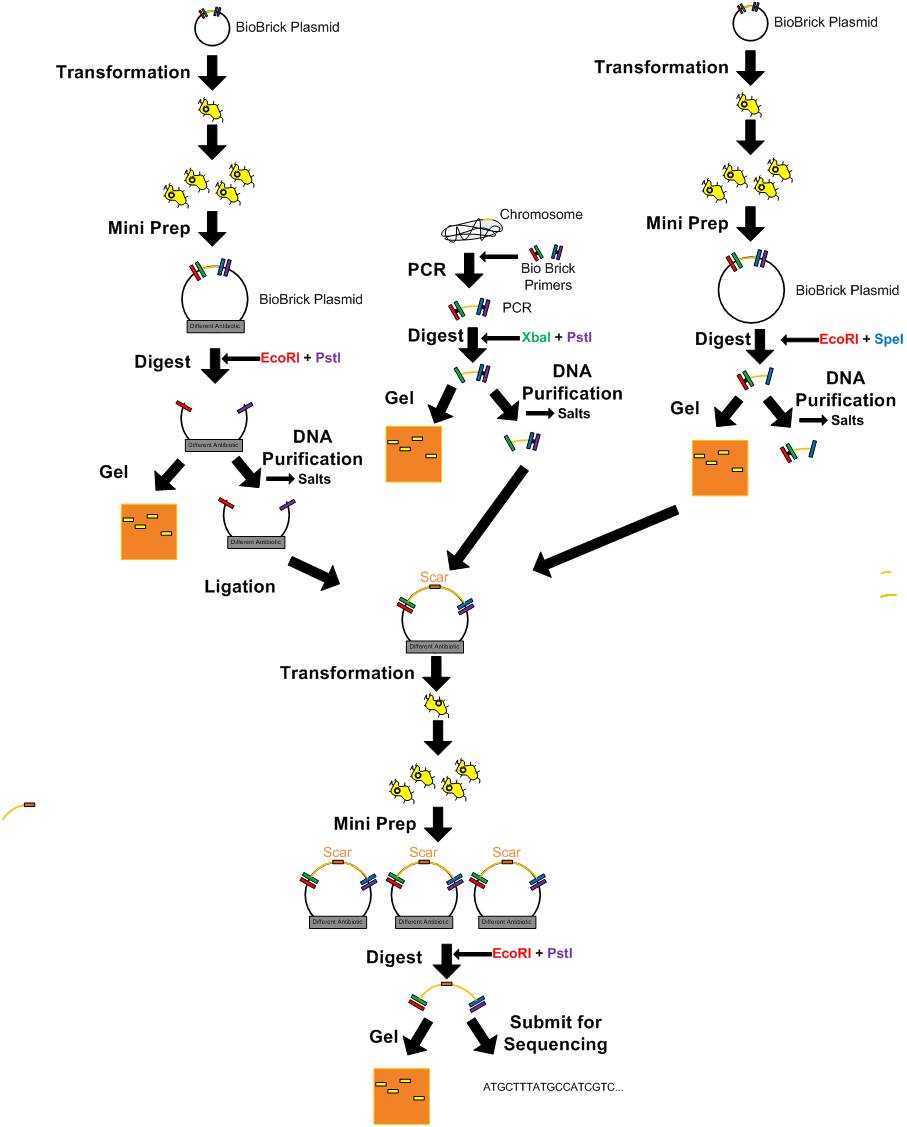
DNA Manipulation
- Getting parts from the 360 well registry plates
- Competent cell preparation
- Heat shock
Transformation-electroporation
- Getting parts from the 360 well registry plates
- Competent cell preparation
- Electroporation
Updated T4 DNA Ligase Protocol (Not quick ligase)
- Ligation
- Ginko Bioworks Protocol
- Jeremy Minty Protocol
Group-Specific Protocols
Oil Sands
Revised biofilm assay protocol
 "
"