Check out these important project links!

|

|

|
|---|---|---|
Contents
Compartmentalization Parts
One of the sub-projects for the bioremediation of the tailings ponds is to create synthetic microcompartments that we can then use to isolate various pathway within an Escherichia coli cell. To do this we need to have a microcompartment as well as a means to characterize the compartment so that the system can be optimized. Here are the experiments we have performed so far towards the characterization of the microcomparments.
Placement of Oligoarginine Tail on Proteins
Characterized Parts
BBa_K249004
BBa_K249005
BBa_K331030
BBa_K331031
Hypothesis
The placement of an oligoarginine sequence at the N-terminus of a protein will destabilize the protein in vivo.
Introduction
The long term goal of our team is to utilize an oligoarginine tail to specifically target enzymes into a microcompartment composed of modified lumazine synthase subunits. While conducting background research on the project, we came upon data originally reported by Bachmair et al.1 suggesting that the identity of the amino acid at the N-terminus of a protein is related to its half-life, and mostly notably, that arginine residues at the are destabilizing. This data suggests that by placing an arginine at the N-terminus of a protein to be targeted into a lumazine synthase microcompartment would cause degradation of our protein before it can be moved into the microcompartment.
We chose to investigate the how the placement of an oligoarginine sequence affects the stability of the protein to which it is fused.
Method
In order to further characterize the C-terminal and N-terminal oligoarginine tag (BioBricks BBa_K249005 and BBa_K249004 respectively) and investigate the effect their placement on protein stability, yellow fluorescent proteins (YFP) with the oligoarginine fused to either the C-terminus (BBa_K331023) or N-terminus (BBa_K331022) (and preceded by a ribosomal binding site – BBa_B0034) were synthesized. We used our Red/White 3-Antibiotic assembly method to add a tetracycline repressible promoter (BBa_R0010) for constitutive expression of the fusion protein. This addition generated BioBricks BBa_K331031 and BBa_K331030 for the C-terminal tagged and N-terminal tagged YFP respectively.
The BioBrick containing plasmid was transformed into Escherichia coli DH5α cells. These cells were grown to an OD600 of approximately 0.7, and diluted 1:10 with MilliQ H2O immediately prior to analysis by fluorescent spectroscopy.
This dilution of cells was excited at 517 nm, and the emission spectra was read from 522 nm to 650 nm. Fluorescence at 524 nm (emission maxima of YFP) of control cells (Escherichia coli DH5α), N-terminal tagged, and C-terminal tagged YFP were compared.
Results
N-terminal tagged YFP did not have substantially more fluorescence than control cells. Cells expressing C-terminal tagged YFP had ten times more fluorescence than control cells and cells expressing N-terminal tagged YFP.
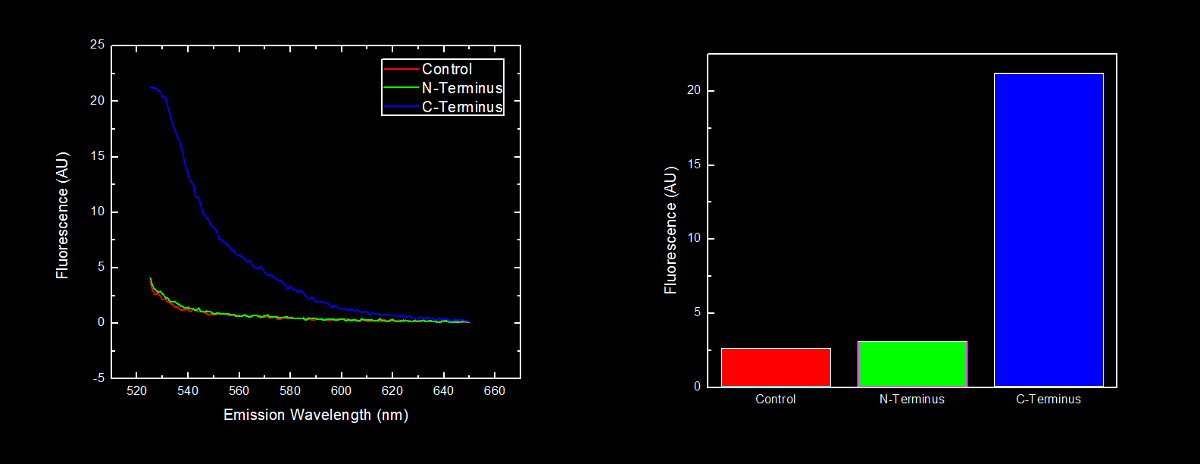
Conclusion
Our results are consistent with the data reported by Bachmair et al. in that the placement of arginine residues at the N-terminus of our YFP results in no observable fluorescence over control cells. Assuming that transcription of this K331030 and K331031 are equivalent, these data suggest that the N-terminal oligoarginine is reducing the half-life of the protein to which it is fused, ie YFP.
Reference
1Bachmair A., Finley D., Varshavsky A. In Vivo Half-Life of a Protein Is a Function of Its Amino-Terminal Residue. Science 234. 4773 179-186.