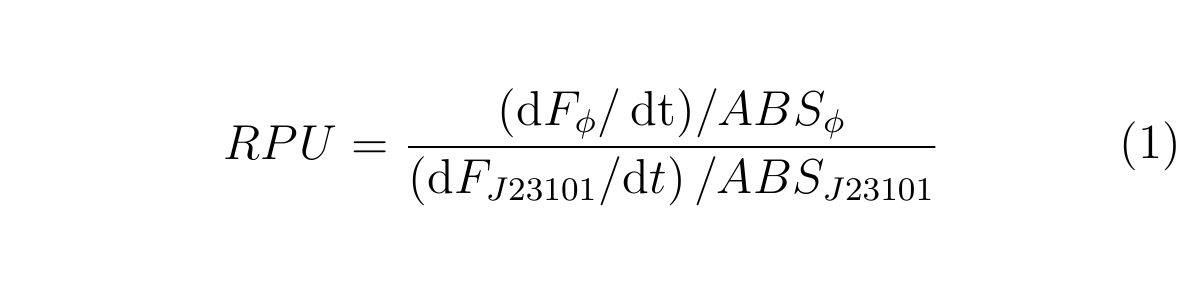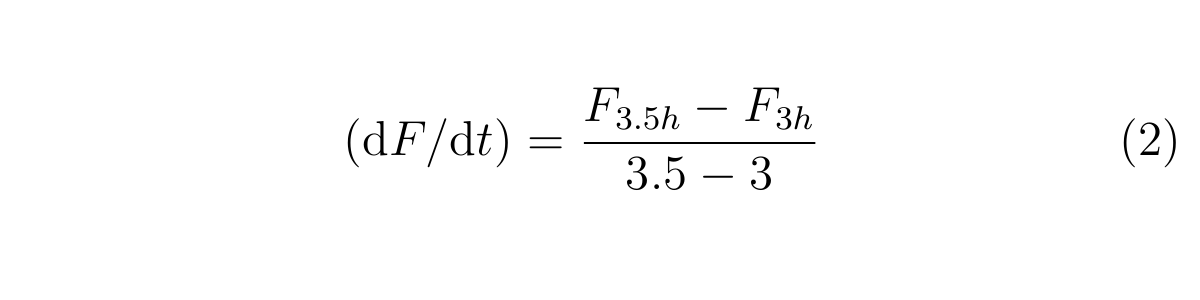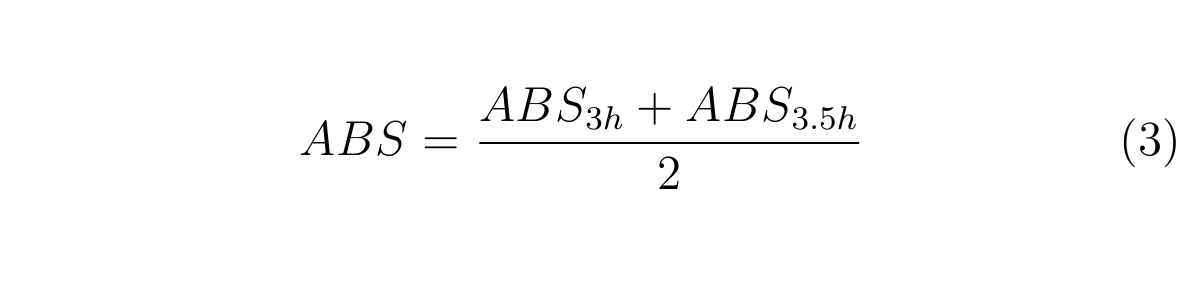Team:Kyoto/Project/Goal A
From 2010.igem.org
(→Method) |
(→Method) |
||
| Line 20: | Line 20: | ||
We picked up three colonies from each plate, and cultivated them in M9 medium supplemented with 50 ug/mL of kanamycine at 37°C for about 16 hours. The precultures were diluted to 1:100 in pre-warmed fresh M9 medium supplemented with 50 ug/mL of kanamycine and incubated with shake at 37°C. After 3h or 3.5h, we measured OD600 followed by GFP fluorescence of the culture. | We picked up three colonies from each plate, and cultivated them in M9 medium supplemented with 50 ug/mL of kanamycine at 37°C for about 16 hours. The precultures were diluted to 1:100 in pre-warmed fresh M9 medium supplemented with 50 ug/mL of kanamycine and incubated with shake at 37°C. After 3h or 3.5h, we measured OD600 followed by GFP fluorescence of the culture. | ||
We measured the GFP fluorescence of some samples after cell was lyzed by the cell lysis reagent and collect the supernatant which contains GFP protein. Unfortunately, we run short of the lysis reagent, the other samples are measured wtiohut using cell lysis. | We measured the GFP fluorescence of some samples after cell was lyzed by the cell lysis reagent and collect the supernatant which contains GFP protein. Unfortunately, we run short of the lysis reagent, the other samples are measured wtiohut using cell lysis. | ||
| + | [[Image:KyotoFigA005.png|600px]] | ||
To know more detail procedures, go [[Team:Kyoto/Protocols|Protocols]]. | To know more detail procedures, go [[Team:Kyoto/Protocols|Protocols]]. | ||
Revision as of 00:03, 28 October 2010
Contents |
Goal A: Characterization of R0011, a strong lactose promoter
Introduction
We used <partinfo>R0011</partinfo> [learn more], a lactose promoter, for the lysis box.
Because part parameters of R0011 are partially unclear [1], we measured the promoter activity of the R0011 at various concenstrations of IPTG in order to make the relationship between the activity of lysis cassette and the expression leves of its promoter.
We characterize R0011 by Relative Promoter Unit (RPU) [learn more], bacause absolute promoter activity depends on test conditions and measurement instruments. RPU can reduce this Coefficient of variation (CV) from 39.1% to17.5% [2]. Therefore RPU can make it easier for us to share the data of promoter activity and use biobricks.
Method
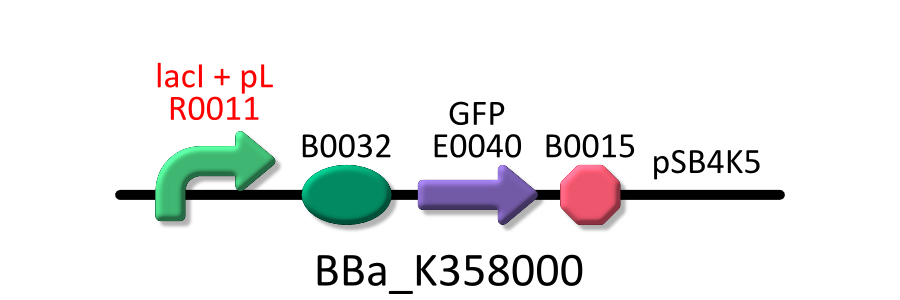
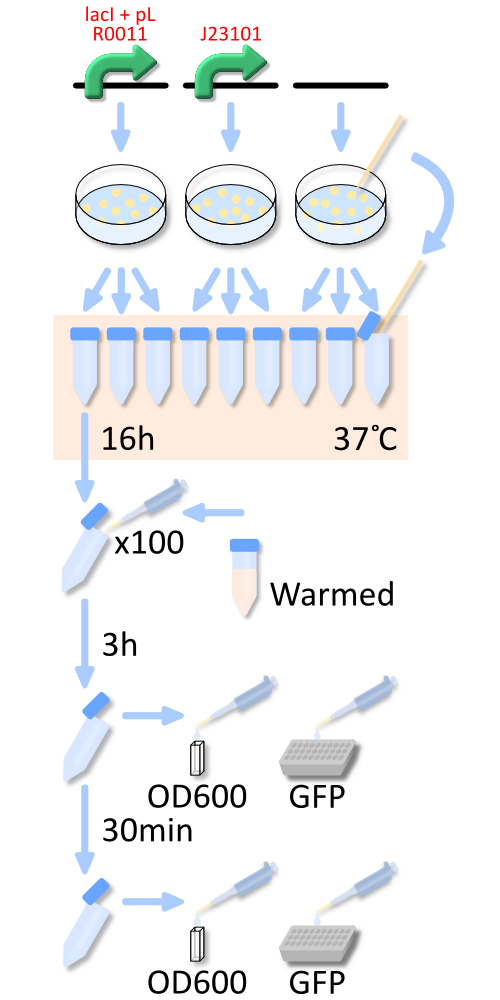
In order to measure RPU of R0011, E. coli host, KRX was transformed with BBa_K358000 (R0011 lac promoter), BBa_K358001 (standard promoter), or pSB4K5 (mock). The reason why we used KRX is that lacI repressor is over expressed in KRX and lacI can repress lactose promoter fully at low IPTG concentration [3].
In order to measure RPU, we must use two types of E.coli transformed with the test promoter circuit and the standard promoter circuit respectively to compare absolute activity of test promoter with that of the standard. BBa_K358000 has the lactose promoter (R0011) immediately upstream of GFP, and BBa_K358001 has the standard promoter (J23101). We constructed the both parts with a low copy number plasmid, pSB4K5, because lacI cannot repress lactose promoter completely even in the absence of IPTG if a high copy number plasmid is used. The E.coli transformed with pSB4K5 was also used for correcting the autofluorescence signal.
We picked up three colonies from each plate, and cultivated them in M9 medium supplemented with 50 ug/mL of kanamycine at 37°C for about 16 hours. The precultures were diluted to 1:100 in pre-warmed fresh M9 medium supplemented with 50 ug/mL of kanamycine and incubated with shake at 37°C. After 3h or 3.5h, we measured OD600 followed by GFP fluorescence of the culture.
We measured the GFP fluorescence of some samples after cell was lyzed by the cell lysis reagent and collect the supernatant which contains GFP protein. Unfortunately, we run short of the lysis reagent, the other samples are measured wtiohut using cell lysis.
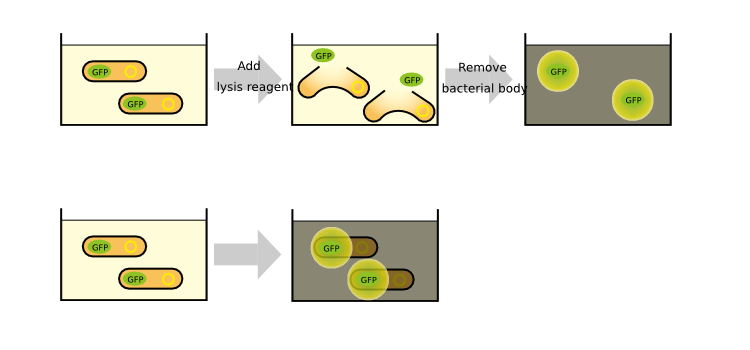
To know more detail procedures, go Protocols.
We measure OD every 30 minutes after dilution to ensure that cells in log phase growth.
RPU is calculated as follows.
We also use these rough calculations;
Here, F3h and F3.5h are the GFP fluorescence measured at 3h and 3.5h, respectively,
ABS3h and ABS3.5h are OD600 of the culture at 3h and 3.5h, respectively.
From the experimental data for cell growth from 2h to 4h after dilution, we assumed cell growth from 3h to 3.5h after dilution can be approximated as linear. Thus, the mean ABS can be calculated as described in (3). If GFP concentration in cell is in steady states, the increase of GFP fluorescence depends on only cell growth. We used the first approximation based on such assumptions to calculate RPU.
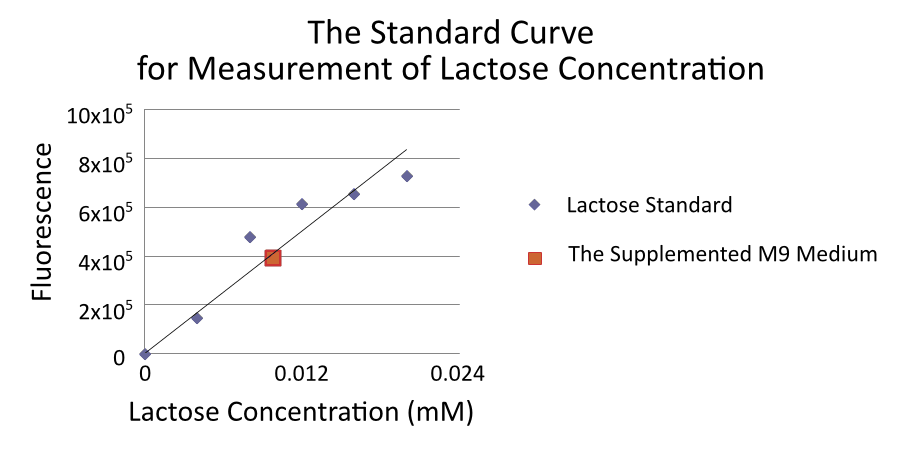
Measurement of lactose concentration is done by using Lactose Assay Kit (BioVison) [6].
Result
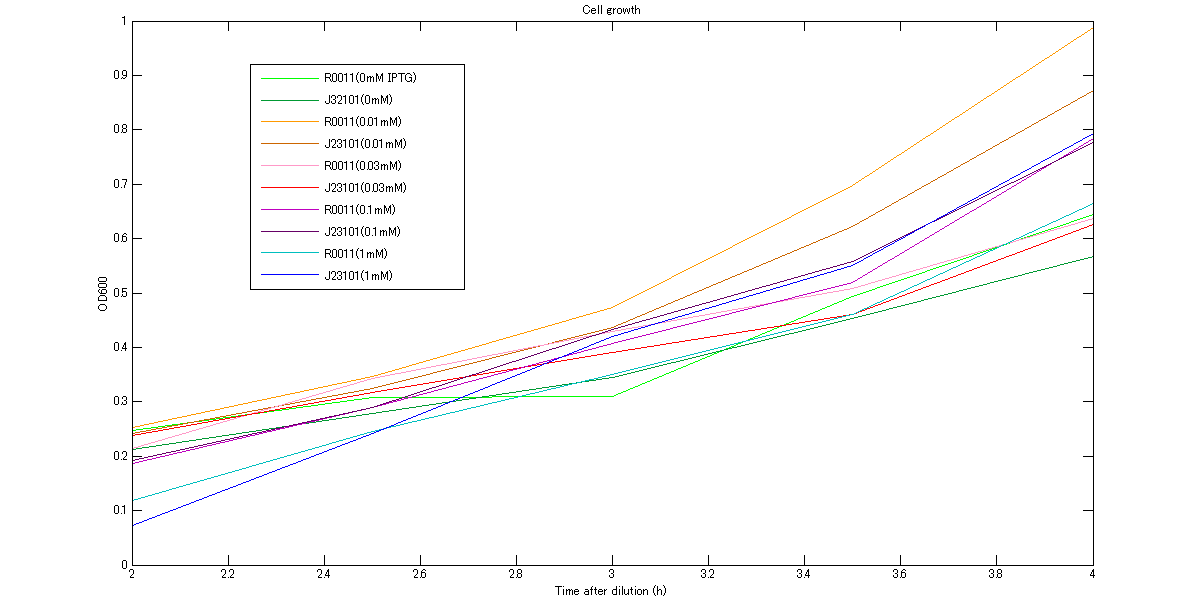
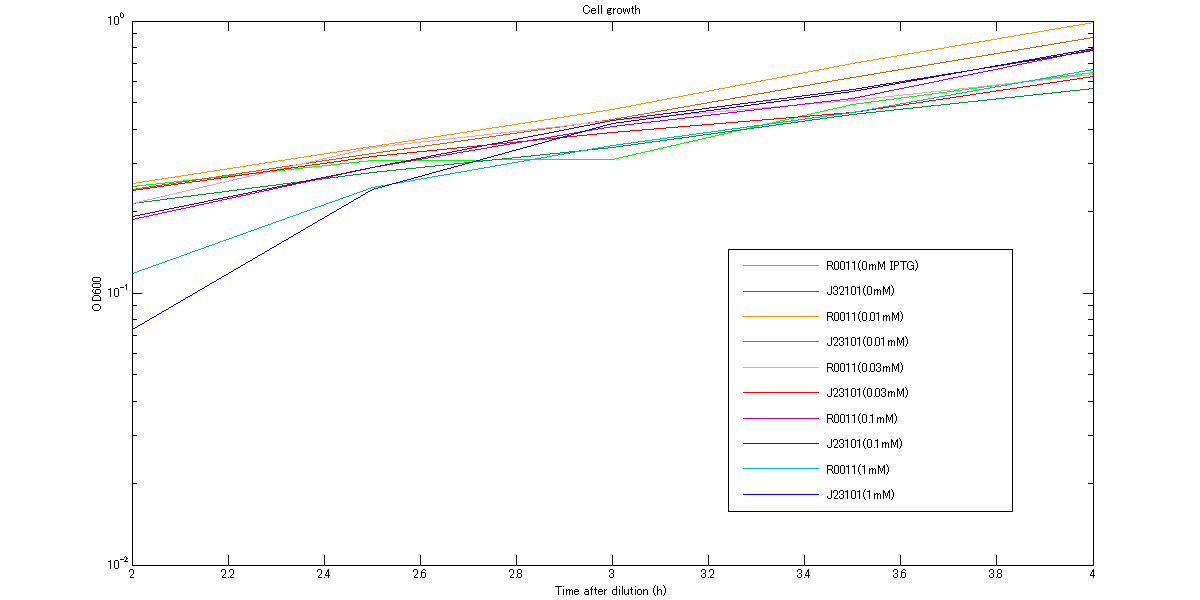
At first, we measured cell growth from 2h to 4h to comfirm our assumption that cells are in log phase growh when we measure RPU. The growth curves are shown in figures below. These graphs indicate that cells were in log phase growth and cell growth from 3h to 3.5h after dilution can be approximated as linear.
Figure1. The growth curves of the cells at various concentrations of IPTG between 2h and 4h. The vertical axis is linear.
Figure2. The growth curves of the cells at various concentrations of IPTG between 2h and 4h. The vertical axis is logarithm.
Thus, we determined RPUs by using equation 1 approximated with 2 and 3. The determined RPUs are shown in table1.
Table1. RPU of R0011 at various concnetaraitons of IPTG
| IPTG (mM) | RPU | |||||
|---|---|---|---|---|---|---|
| A | B | C | Average | Stdev | CV | |
| 0 | * | 0.0122 | 0.0147 | 0.0134 | 0.00821 | 0.611 |
| 0.01 | 0.0191 | 0.00963 | 0.00244 | 0.0104 | 0.0084 | 0.806 |
| 0.03 | 0.177 | 0.155 | 0.198 | 0.174 | 0.0173 | 0.0997 |
| 0.1 | 0.205 | 0.191 | 0.269 | 0.221 | 0.0416 | 0.188 |
| 0.3 | 0.938 | 0.955 | 0.955 | 0.949 | 0.00981 | 0.0103 |
| 0.8 | 1.44 | * | 1.21 | 1.37 | 0.161 | 0.122 |
| 1.0 | 1.60 | 1.58 | 1.65 | 1.65 | 0.106 | 0.0640 |
| 2.0 | 1.49 | 1.83 | 1.39 | 1.57 | 0.233 | 0.148 |
- *One of RPU in IPTG 0mM couldn’t be measured. One of RPU in IPTG 0.8mM couldn't also be measured.
- *In this table, RPU in 0, 0.01, 0.1, 1.0 mM IPTG is measured after cell lyzed by the cell lysis reagent and RPU in 0.05, 0.3, 0.8, 2.0 mM IPTG is measured without cell lysis.
We made the model about the activity of R0011 and decided parameters from the result to estimate RPU of R0011 at IPTG concentrations we couldn’t measure. The result is shown figure3.
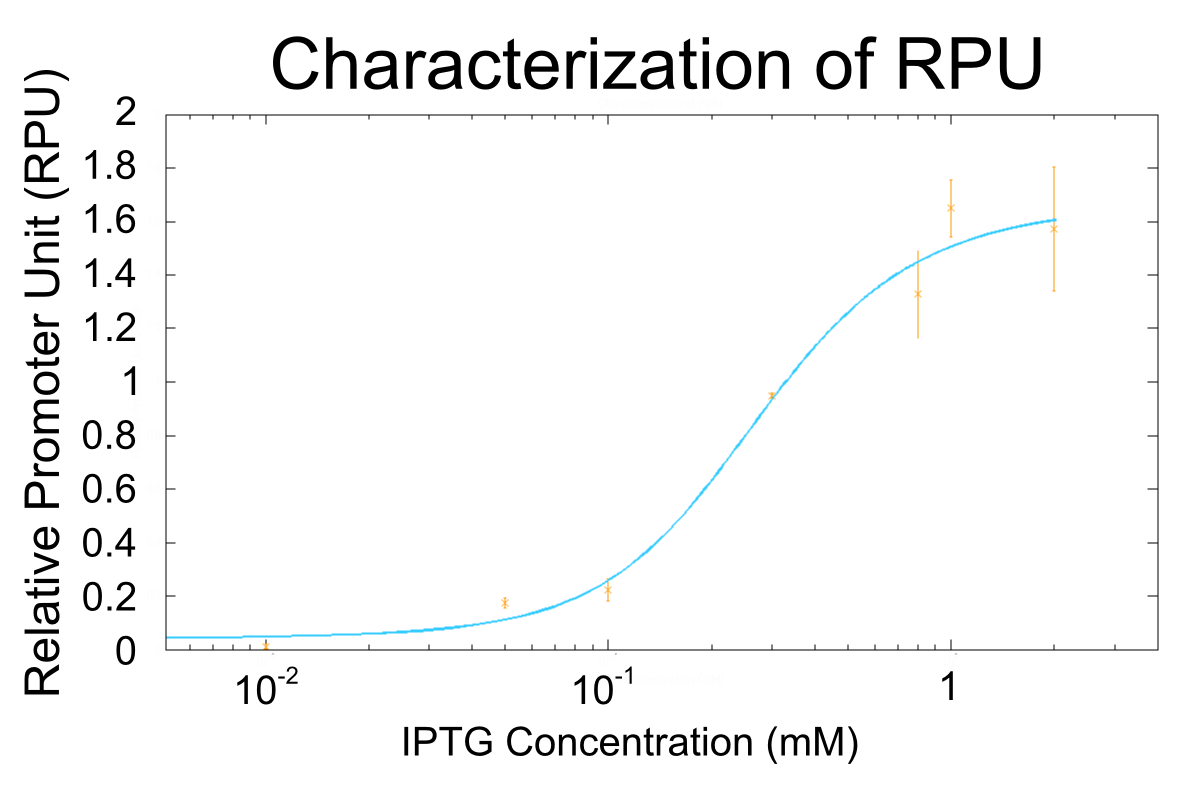
Figure3. The RPUs at various concentrations of IPTG. The blue line indicates the fit/calculated curve predicted by our model. The data represent the mean +/- s.d. obtained by triplicate experiments,
To know more about this model, go Modelling.
Discussion
The result indicates that the maximum activity of R0011 is about 1.6 RPU and the minimum is less than 0.01 RPU and that R0011 has wide range of activity (the maximum activity of R0011 is at least about 160 fold greater than the minimum activity at least). The maximum activity of R0011, 1.6 RPU agrees with the previous data [4]. The wide range of activity of RPU can change the effect of lysis cassette variously, and it is useful to characterize lysis cassette.
The graph of cell growth indicates that cells in log phase growth at 3h and 3.5h after dilution. This means that time of measurement for RPU is correct. In this characterization, there is two way to measure RPU, one is measure GFP fluorescence after cell lysed and the other is measure GFP fluorescence without cell lysed. We think both measurements is valid because RPU of R0011 with 1mM IPTG measured with cell lysis corresponds to the previous data [4] and the way of measurement of RPU without cell lysed is recommended one by the previous igem team [5].
By the way, We found that supplemented M9 medium was contaminated by approximately 0.01mM lactose.
We thought that lactose in the supplemented M9 medium comes from casamino acids because casamino acids made from milk which contains lactose. Therefore, we tried to culture E.coli and measure RPU without the casamino acids because lactose in the supplemented M9 medium can induce R0011. However, without casamino acids, the growth rate of E.coli declines and we could not measure RPU. The all RPU data was measured with casamino acids. Accordingly, the real value for RPU of R0011 at low IPTG concentration may be lower than the value gained from this experiment. Measurement of lactose concentration is done by using Lactose Assay Kit (BioVison) .[6] CV in 0mM IPTG is so high, 0.611, and CV in 0.01mM IPTG is also high, 0.806. We cannot trust RPU in 0mM and 0.01mM ITPG so much.
Finally, we could characterize R0011 at high IPTG concentration correctly. However, characterization of R0011 at low IPTG concentration may be required additional measurements.
Reference
- http://partsregistry.org/Part:BBa_R0011
- Jason R Kelly et al. ”Measuring the activity of BioBrick promoters using an in vivo reference standard.” Journal of Biological Engineering 2009, 3:4
- http://www.promega.com/pnotes/94/14410_27/14410_27.pdf
- http://partsregistry.org/Part:BBa_R0011:Experience
- https://2009.igem.org/Team:UNIPV-Pavia/Methods_Materials/Fluorescence#Fluorescence_and_water_dispensation
- http://www.biovision.com/manuals/K624-100.pdf
 "
"