Team:Kyoto/Notebook
From 2010.igem.org
Index
Notebook
Tuesday, July 20
By: Wataru, Tomo, Yuki, Kazuya, Ken, Makoto
1. Solubilization of antibiotics.
For Ampicillin(Amp), add 1.0g Amp to 20ml MilliQ (final concentration is 50mg/ml). For Kanamycin(Kan), add 0.5g Kan to 10ml MilliQ (final concentration is 50mg/ml). Dispense 1.1ml of the solution into 1.5ml tubes and store in the freezer (-20℃).
2. Make plates for LB (Amp+) and LB (Kan+).
3. Transformation of iGEM Parts.
| Name | Well | Sample (µl) | Competent Cells (µl) | Total (µl) | Plate | Incubation | Result |
|---|---|---|---|---|---|---|---|
| <partinfo>J23100</partinfo> | 1-18-C | 1 | 20 | 21 | LB (Amp+) | At 37℃ 7/20 20:50 - 7/21 17:00 | ○ |
| <partinfo>J23105</partinfo> | 1-18-M | 1 | 20 | 21 | ○ | ||
| <partinfo>J23116</partinfo> | 1-20-M | 1 | 20 | 21 | ○ | ||
| <partinfo>R0011</partinfo> | 1-6-G | 1 | 20 | 21 | ○ | ||
| <partinfo>E0840</partinfo> | 1-12-O | 1 | 20 | 21 | ○ | ||
| <partinfo>J06702</partinfo> | 2-8-E | 1 | 20 | 21 | ○ | ||
| <partinfo>pSB4K5</partinfo> | 1-5-G | 1 | 20 | 21 | × | ||
| <partinfo>B0015</partinfo> | 1-23-L | 1 | 20 | 21 | LB (Kan+) | × |
A vector of "pSB4K5" is Kanamycin-resistance, however, we plated it to LB plate (Amp+). And We didn't pre-culture "B0015" despite its vector is Kanamycin-resistance. So, it was predicted that we will fail the transformation of "pSB4K5" and "B0015".
Wednesday, July 21
By: Wataru, Ken, Makoto, Takuya Yamamoto
1. Culture plates in which colonies was observed at 37℃ from 07/21 20:50 to 07/22 17:00.
2. Make a master plate of the above plates.
3. Retry Transformation of iGEM Parts.
| Name | Well | Sample (µl) | Competent Cells (µl) | Total (µl) | Plate | Incubation | Result |
|---|---|---|---|---|---|---|---|
| <partinfo>pSB4K5</partinfo> | 1-5-G | 1 | 20 | 21 | LB (Kan+) | At 37℃ 7/21 20:50 - 7/22 16:30 | ○ |
| <partinfo>B0015</partinfo> | 1-23-L | 1 | 20 | 21 | ○ |
4. PCR for S-R-Rz/Rz1 and S
Dilute λDNA (0.5µg/µl) 100 times with MilliQ. The final concentration of template λDNA was 5ng/µl.
| No. | Water | 25mM MgSO4 | 2mM dNTPs | 10xBuffer for KOD Plus ver.2 | TemplateDNA (5ng/µl) | Primer Forward (10µM) | Primer S-R-Rz/Rz1 Reverse (10µM) | Primer S Reverse (10µM) | KOD Plus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28µl | 3µl | 5µl | 5µl | 5µl | 1.5µl | 1.5µl | - | 1µl | 50µl |
| 2 | 28µl | 3µl | 5µl | 5µl | 5µl | 1.5µl | 1.5µl | - | 1µl | 50µl |
| 3 | 28µl | 3µl | 5µl | 5µl | 5µl | 1.5µl | - | 1.5µl | 1µl | 50µl |
| 4 | 28µl | 3µl | 5µl | 5µl | 5µl | 1.5µl | - | 1.5µl | 1µl | 50µl |
| 5 | 28µl | 3µl | 5µl | 5µl | 5µl | 1.5µl | 1.5µl | - | 1µl | 50µl |
| 6 | 28µl | 3µl | 5µl | 5µl | 5µl | 1.5µl | 1.5µl | - | 1µl | 50µl |
| 7 | 28µl | 3µl | 5µl | 5µl | 5µl | 1.5µl | - | 1.5µl | 1µl | 50µl |
| 8 | 28µl | 3µl | 5µl | 5µl | 5µl | 1.5µl | - | 1.5µl | 1µl | 50µl |
Forward Primer of S-R-Rz/Rz1 and S is common. PCR condition: 94℃ x 2min, (98℃ x 10sec, 55℃ x 30sec, 68℃ x 1min) x 30cycles, 4℃ forever.
Thursday, July 22
By: Wataru
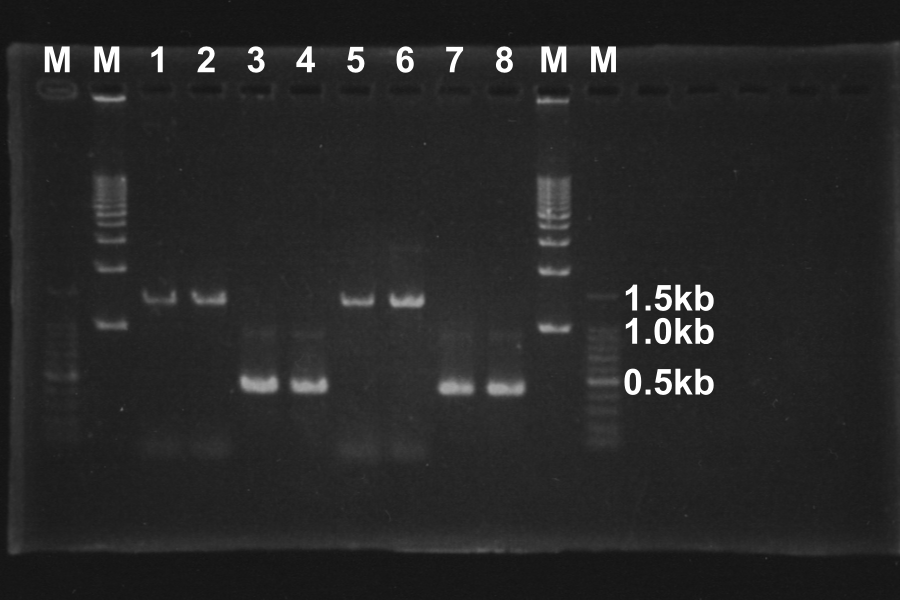
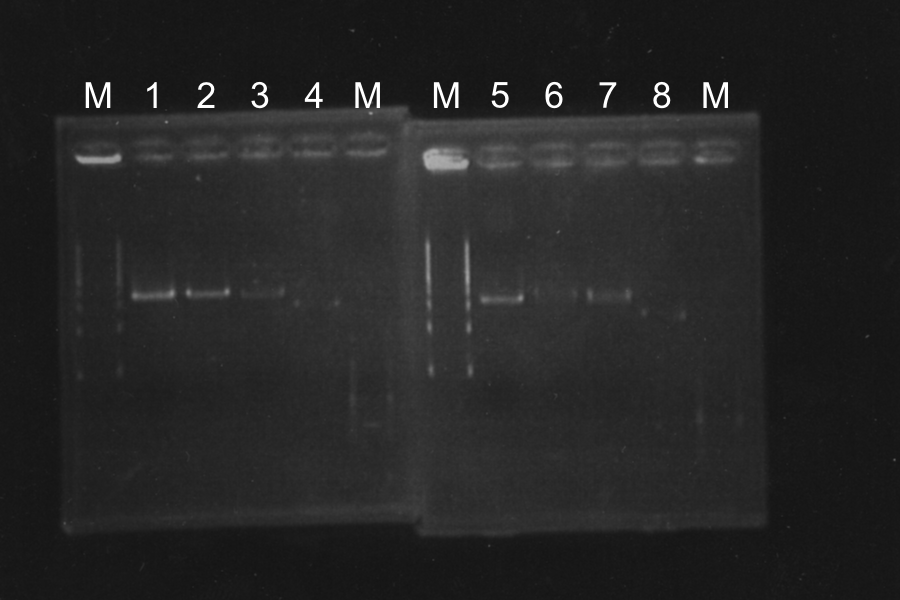
1. Electrophoresis of the PCR products for 40min.
Length of S and S-R-Rz/Rz1 is 370bp, 1300bp, so PCR succeeded.
2. Miniprep of iGEM Parts.
| Name | Concentration(ng/µl) |
|---|---|
| <partinfo>J23100</partinfo> | 18.5 |
| <partinfo>J23105</partinfo> | 12.5 |
| <partinfo>J23116</partinfo> | 14.6 |
| <partinfo>R0011</partinfo> | 8.6 |
| <partinfo>E0840</partinfo> | 12.1 |
| <partinfo>J06702</partinfo> | 14.7 |
The concentration of all samples was very week. Probably our shaking incubation was week.
3. Culture plates and make master plates of <partinfo>pSB4K5</partinfo> and <partinfo>B0015</partinfo> from 7/22 17:00 to 7/23 10:00.
Friday, July 23
By: Wataru, Tomo, Makoto
1. Miniprep of iGEM Parts.
| Name | Concentration(ng/µl) |
|---|---|
| <partinfo>pSB4K5</partinfo> | 79.2 |
| <partinfo>B0015</partinfo> | - |
We lost <partinfo>B0015</partinfo> by our mistake. The concentration of <partinfo>pSB4K5</partinfo> is high, so this condition of shaking incubation is moderate.
2. Picked up 1, 3, 5, 7 of the products of PCR, and purified by PCR-purification.
| Sample | Concentration (ng/µl) | New Name | |
|---|---|---|---|
| 1 | 18.6 | - | |
| 3 | 77.6 | S1 | |
| 5 | 33.6 | - | |
| 7 | 65.4 | S2 |
The concentration of sample number 1 and 5, the PCR products of S-R-Rz/Rz1, is week, so we desided to retry PCR.
3. Retry of PCR of S-R-Rz/Rz1.
| Sample | Water | 25mmol/l MgSO4 | 2mmol/l dNTPs | 10×Buffer for KOD plus ver.2 | Template DNA (5ng/µl) | Primer S-R-Rz/Rz1 Forward (10µmol/l) | Primer S-R-Rz/Rz1 Reverse (10µmol/l) | KOD plus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 28µl | 3 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 2 | 28 | 3 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 3 | 26.5 | 4.5 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 4 | 26.5 | 4.5 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 5 | 25 | 6 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 6 | 25 | 6 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
PCR condition : 94℃ x 2min, (98℃ x 10sec, 55℃ x 30sec, 68℃ x 1min) x 30cycles, 4℃ forever.
4. Digested <partinfo>J06702</partinfo> by EcoRI, XbaI, SpeI, PstI to check function of our Restriction enzymes.
| Sample | 10xBuffer | BSA | Enzyme | MilliQ | Total | Incubation |
|---|---|---|---|---|---|---|
| 1 | 5µl | 1 | EcoRI 0.1 | 3.6 | 10 | At 37℃ 7/23 18:00 - 7/23 18:30 |
| 2 | 5 | 1 | XbaI 0.1 | 3.6 | 10 | |
| 3 | 5 | 1 | SpeI 0.1 | 3.6 | 10 | |
| 4 | 5 | 1 | PstI 0.1 | 3.6 | 10 | |
| 5 | 5 | 1 | - | 3.7 | 10 |
5. Electrophoresis of above sample for 35min.
Comparison to sample 5(control, circular DNA), the bands of sample 1, 2, 3, 4 was shifted. The DNA of sample 1, 2, 3, 4 was linearized by Restriction enzymes. So, our restriction enzymes work correctly.
6. To insert S gene to GFP, we digested the PCR products of S gene by EcoRi and SpeI, and GFP by EcoRl and XbaI.
| Sample | 10×Buffer | Enzyme 1 | Enzyme 2 | MilliQ | Total | Incubation | |
|---|---|---|---|---|---|---|---|
| S1 | 11µl | 5 | EcoRI 0.2 | SpeI 0.2 | 33.6 | 50 | At 37℃ for 2h |
| S2 | 11 | 5 | EcoRI 0.2 | SpeI 0.2 | 33.6 | 50 | |
| <partinfo>E0840</partinfo>(GFP) | 45 | 5 | EcoRI 0.2 | XbaI 0.2 | 0 | 50 |
After PCR purification, evaporated them and diluted 3ul.
7. Ligated over night.
| Sample | Vector | Insert | Ligation High | Total |
|---|---|---|---|---|
| S-GFP1 | <partinfo>E0840</partinfo> 0.5µl | S1 0.5 | 1 | 2 |
| S-GFP2 | <partinfo>E0840</partinfo> 0.5 | S2 0.5 | 1 | 2 |
Monday, July 26
By: Wataru, Tomonori, Makoto
1. Electrophoresis of PCR products
At the condition 4 (4.5µl MgSO4) and 6 (6µl MgSO4), S-R-Rz/Rz1 is amplified very much. So we decided to use them.
2. PCR purification
| Sample | Concentration (ng/µl) | New Name |
|---|---|---|
| 4 | 51.6 | |
| 5 | 59.3 | |
| 6 | 59.6 |
3. Transformation of iGEM Parts
| Name | Well | Sample (µl) | Competent Cell (µl) | Total (µl) | Plate | Incubation | Result |
|---|---|---|---|---|---|---|---|
| 1-12-M | 1 | 20 | 21 | LB (Amplicillin+) | At 37℃ 7/26 - 7/27 | × | |
| 2-17-F | 1 | 20 | 21 | LB (Kanamycin+) | × | ||
| 1-5-E | 1 | 20 | 21 | × |
4. Culture of 1-6-G, 1-12-O, and 1-23-L
Tuesday, July 27
By: Wataru, Tomo, Kazuya, Ken, Naoi
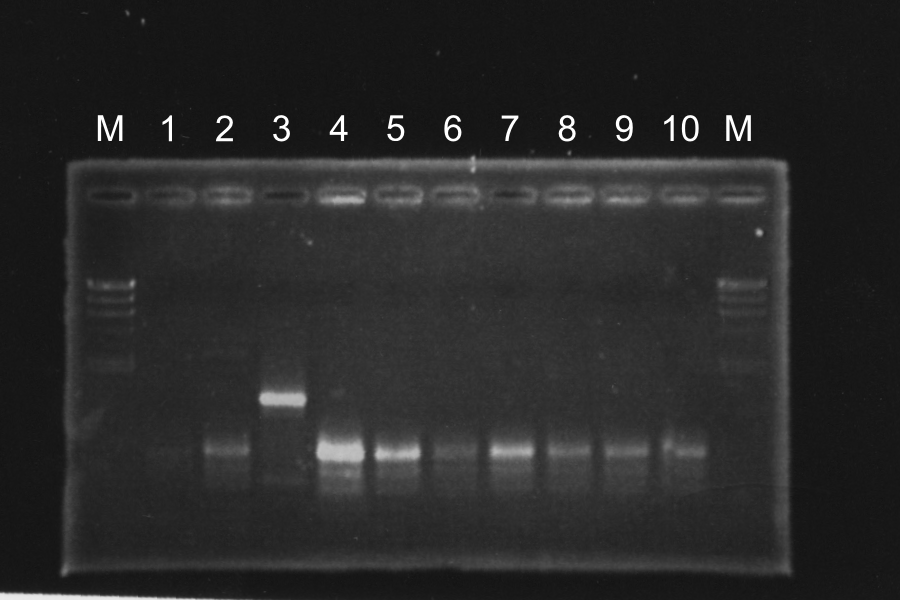
1. Colony PCR of S-E840
To check that S GFP is correctly inserted, we did colony PCR. Electrophoresis was done for 35min.
| Marker | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | + | - | Marker |
| 1kb | S-E08401 | S-E08402 | E0840 | None | 100bp |
File:KyotoExp100727.png As a result, 1,3,5,6,11,12,13 are inserted S gene correctly. So, we decided to use 6 as S-E08401 and 11 as S-E08402.
2. Miniprep
| Sample number | Concentration(ng/µL) |
|---|---|
| 1-6-G | 26.9 |
| 1-23-L | 120.0 |
| 1-12-O | 120.1 |
3. Restriction Digestion
| Sample volume | 2 buffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | |||
|---|---|---|---|---|---|---|---|---|---|
| 1-23-L | 30 | 5 | 0.5 | EcoRI | 0.4 | XbaI | 0.3 | 13.7 | 50 |
| 4.5② | 40 | 5 | 0.5 | 0.4 | 0.4 | 3.8 | 50 | ||
| 6② | 40 | 5 | 0.5 | 0.4 | 0.4 | 3.8 | 50 | ||
Incubate 37℃ 16:45~18:00
4. Ligation
5. Transformation
Wednesday, July 28
1. Result of Transformation
| SRRz①-DT | Many colonies |
| SRRz②-DT |
2. Deletion PCR
To delete functional domain of S gene, we did deletion PCR.
Miniprep
| Sample number | Concentration(ng/µ) |
|---|---|
| S-E840① | 95.5 |
| S-E840② | 98.6 |
Diluted S-GFP① and S-GFP② 20 times with water, and used as template DNA.
Deletion PCR
To delete functional region of S gene, we did deletion PCR.
| Water | 25mM MgSO4 | 2mM dNTPs | 10x buffer for KOD Plus ver.2 | Primer Deletion F(10µM) | Primer Deletion R(10µM) | Template S-E840① | Template S-E840② | KOD Plus ver.2 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Δ1-1 | 28 | 3 | 5 | 5 | 1.5 | 1.5 | 5 | - | 1 | 50 |
| Δ1-2 | 28 | 3 | 5 | 5 | 1.5 | 1.5 | 5 | - | 1 | 50 |
| Δ2-1 | 28 | 3 | 5 | 5 | 1.5 | 1.5 | - | 5 | 1 | 50 |
PCR program
| 94℃ | 2min | |
| 98℃ | 10sec | 35 cycles |
| 55℃ | 30sec | |
| 68℃ | 4min | |
| 4℃ | forever |
3. RE
To check function of our Restriction enzymes, we digested S-E840① and S-E-840② by DpnI.
| Sample | fast digestion buffer | DpnI | MilliQ | Total | |
|---|---|---|---|---|---|
| S-E840① | 3 | 1 | 0.1 | 5.8 | 10 |
| S-E840② | 3 | 1 | 0.1 | 5.8 | 10 |
Electrophoresis
Gel: Agarose Time: 35min Voltage: 100V Maker: 1K 100
1 1k marker 2 not digested S-E840① 3 not digested S-E840② 4 digested S-E840① 5 digested S-E840② 6 100bp marker
1k 1 2 3 4 100
Discussion
DpnI works correctly
Thursday, July 29
RE
| Sample volume | Fastdigestion buffer | Enzyme 1 | MilliQ | Total | |
|---|---|---|---|---|---|
| Δ1-1 | 50 | 6 | DpnI 0.2 | 3.8 | 60 |
| Δ2-1 | 50 | 6 | DpnI 0.2 | 3.8 | 60 |
Incubate 7/29 9:40~7/29 11:00
Ligation and Pospholylation
| Sample | MilliQ | Ligation High | T4 Kinase | Total | |
|---|---|---|---|---|---|
| Δ1-1 | 2 | 7 | 5 | 1 | 15 |
| Δ2-1 | 2 | 7 | 5 | 1 | 15 |
Incubate 7/29 11:30~7/29 13:00
Transformation
| Sample | Conc(/µL) | Sample Volume(µL) | Competent Cell(µL) | Total | Plate | Incubation |
|---|---|---|---|---|---|---|
| Δ1-1 | - | 3 | 30 | 33 | LB amp | 7/29~7/30 |
| Δ1-1 | - | 3 | 30 | 33 |
Friday, July 30
Result of transformation of Δ1 and Δ2 Many colonies are observed.
Monday, August 2
By: Wataru, Ken
1. Miniprep
| Sample number | Concentration(ng/µL) |
|---|---|
| Δ1 | 52.7 |
| Δ2 | 54.4 |
| Δ3 | 89.5 |
| pSB4K5 | 50.7 |
| LacP | 18.6 |
2. PCR and RE of E240
E240 is very important parts to measure RPU of promoters in iGEM. However, we failed to transfect it to E.coli from parts kit of iGEM. So we decided to amplify this parts by PCR.
| Water | 25mM MgSO4 | 2mM dNTPs | 10xBuffer for KOD Plus ver.2 | Primer VF2(10µM) | Primer VR(10µM) | Template E240 | KOD Pllus ver.2 | Total | |
|---|---|---|---|---|---|---|---|---|---|
| E240① | 28 | 3 | 5 | 5 | 1.5 | 1.5 | 5 | 1 | 50 |
| E240② | 28 | 3 | 5 | 5 | 1.5 | 1.5 | 5 | 1 | 50 |
PCR program
| 94℃ | 2min | |
| 98℃ | 10sec | 35 cycles |
| 55℃ | 30sec | |
| 68℃ | 4min | |
| 4℃ | forever |
Electrophoresis
PCR purification
| Sample number | Concentration(ng/µL) |
|---|---|
| E240① | 42.6 |
| E240② | 55.3 |
RE
To insert E240 to pSB4K5 by 3A assembly, we digested the PCR products of E240 by XbaI and PstI
| Sample volume | 2 buffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | |||
|---|---|---|---|---|---|---|---|---|---|
| E240①X-P | 30 | 5 | 0.5 | XbaI | 0.2 | PstI | 0.2 | 14.1 | 50 |
| E240②X-P | 30 | 5 | 0.5 | XbaI | 0.2 | PstI | 0.2 | 14.1 | 50 |
PCR purification
| Sample number | Concentration(ng/µL) | Volume(µL) |
|---|---|---|
| E240①X-P | 21.8 | 40 |
| E240②X-P | 32.4 | 45 |
-20℃ freezer
3. Error PCR
| Water | 25mM MgSO4 | 2mM dNTPs | 10xBuffer for KOD Plus ver.2 | Primer VF2(10µM) | Primer VR(10µM) | Template Δ1 | Template | Template | KOD Pllus ver.2 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔTMD① | 32 | 3 | 5 | 5 | 1.5 | 1.5 | 1 | - | - | 1 | 50 |
| ΔTMD② | 32 | 3 | 5 | 5 | 1.5 | 1.5 | - | 1 | - | 1 | 50 |
| ΔTMD③ | 32 | 3 | 5 | 5 | 1.5 | 1.5 | - | - | 1 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10sec | 20 cycles |
| 68℃ | 4min | |
| 4℃ | forever |
After the digestion by DpnI, we transfected 2µL of sample to 20µL of competent cell.
Tuesday, August 3
1. The result of transformation
| ΔTMD① | Many colonies |
| ΔTMD② | No colony |
| ΔTMD③ | Many colonies |
We picked two colonies from ΔTMD① and ΔTMD③, and cultured 37℃ 8/3~8/4.
2. The construction of ML and MS
Miniprep
| Sample number | Concentration(ng/µL) |
|---|---|
| pSB4K5 | 60.7 |
| LacP | 26.8 |
RE
| Sample volume | 2 buffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | |||
|---|---|---|---|---|---|---|---|---|---|
| LacP | 50 | 6 | 0.6 | EcoRI | 0.2 | SpeI | 0.2 | 3 | 60 |
| pSB4K5(E-P) | 50 | 6 | 0.6 | EcoRI | 0.2 | PstI | 0.2 | 3 | 60 |
| E240①(X-P) | 50 | 6 | 0.6 | XbaI | 0.2 | PstI | 0.2 | 3 | 60 |
| E240②(X-P) | 50 | 6 | 0.6 | XbaI | 0.2 | PstI | 0.2 | 3 | 60 |
Incubate
Purification
PCR purification
| Sample number | Concentration(ng/µL) |
|---|---|
| pSB4K5 E-P | 39.5 |
| E240①X-P | 21.8 |
| E240②X-P | 32.4 |
pSB4K5 E-P is concentrated 10µL and E240①X-P, E240②X-P are concentrated 1µL.
Ethanol precipitation
1-5-G dilute milliQ 2µL
Ligation
| Vector | Insert 1 | Insert 2 | Ligation High | Total | |
|---|---|---|---|---|---|
| ML1 | pSB4K5 E-P 1 | LacI E-S 1 | E240①X-P 1 | 3 | 15 |
| ML2 | pSB4K5 E-P 1 | LacI E-S 1 | E240②X-P 1 | 3 | 15 |
Incubation 17:30~20:20
PCR of J23101-E240
J23101-E240 is important in the measurement of RPU, so we amplified this parts by PCR.
| Water | 25mM MgSO4 | 2mM dNTPs | 10xBuffer for KOD Plus ver.2 | Primer VF2(10µM) | Primer VR(10µM) | Template J23101-E240 | KOD plus ver.2 | Total | |
|---|---|---|---|---|---|---|---|---|---|
| MS① | 32 | 3 | 5 | 5 | 1.5 | 1.5 | 1 | 1 | 50 |
| MS② | 32 | 3 | 5 | 5 | 1.5 | 1.5 | - | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10sec | 30 cycles |
| 55℃ | 30sec | |
| 68℃ | 4min | |
| 4℃ | forever |
PCR purification
| Sample number | Concentration(ng/µL) |
|---|---|
| J23101-E240 | 40.6 |
Discussion J23101-E240(MS) is amplified corrrecly.
RE
| Sample volume | 2 buffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | |||
|---|---|---|---|---|---|---|---|---|---|
| J23101-E240(E-P) | 45 | 6 | 0.6 | EcoRI | 0.2 | PstI | 0.2 | 8 | 60 |
PCR purification
| Sample number | Concentration(ng/µL) | Volume(µL) |
|---|---|---|
| J23101-E240 E-P | 74.1 | 30 |
J23101-E240 is concentrated 7µL
Ligation
| Vector | Insert | Ligation High | Total | |
|---|---|---|---|---|
| MS | pSB4K5 E-P 1 | J23101-E240 E-P 1 | 2 | 4 |
Incubation 20:00~20:30
Transformation
| Sample | Conc(/µL) | Sample Volume(µL) | Competent Cell(µL) | Total | Plate | Incubation |
|---|---|---|---|---|---|---|
| ML1 | - | 1 | 20 | 21 | LB kan | 8/3~8/4 |
| ML2 | - | 1 | 20 | 21 | ||
| MS | - | 1 | 20 | 21 |
Thursday, August 5
1. Result of transformation
| ML1 | Many colonies |
| ML2 | |
| MS |
ML1 and ML2
pSB4K5 is inserted RFP generator. We didn't distinguish this inserted parts from low copy plasmid backbone, so self-ligated colony is red. So, white colony is correctly inserted parts. However, white colonies and green colonies are observed in ML1 and ML2 plate. We cultured both white and green colonies.
MS Self-ligated colony is red Many of colonies are red, however, green colonies are observed.
Culture and Master
Green colony ML1-1 ML1-2 ML2-1 MS1-1 MS1-2 MS1-3 White colony ML1-3 ML1-4 ML2-2 ML2-3 ML2-4
J23100 and LacP 8/5~8/6
2. Sequence
| Sample number | Concentration(ng/µL) |
|---|---|
| ΔTMD①A | 28.9 |
| ΔTMD①B | 25.3 |
| ΔTMD③A | 26.6 |
| ΔTMD③B | 24.0 |
As a result, deletion is succeeded, however, point mutation is failed. It is because DpnI is too little to digest all of template DNA.
Friday, August 6
1. Miniprep
ML1-1 ML1-2 ML1-3 ML1-4 ML2-1 ML2-2 ML2-3 ML2-4 MS1 MS2 MS3
2. RE
| Sample volume | 2 buffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | ||
|---|---|---|---|---|---|---|---|---|
| 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
Electrophoresis
1 100bp 2λ 3λ 4100bp 5MS1 6MS2 7MS3 8ML1-1 9ML1-2 10ML1-3 11ML1-4 12ML2-1 13ML2-2 14ML2-3 15ML2-4 16MS1 17MS2
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17
Discussion MS1 and MS2 are inserted correctly. ML1-1 and ML1-2 are inserted correctly. ML2-1 are inserted correctly. White colonies are inserted not lacP but its vector. Top10 we used are deleted Lac operon. Then, correctly inserted parts is green because of the lack of LacI.
Error PCR(Retry)
| Water | 25mM MgSO4 | 2mM dNTPs | 10xBuffer for KOD Plus ver.2 | Primer VF2(10µM) | Primer VR(10µM) | Template ΔTMD failed(50ng/µL) | KOD plus ver.2 | Total | |
|---|---|---|---|---|---|---|---|---|---|
| ΔTMD① | 32 | 3 | 5 | 5 | 1.5 | 1.5 | 1 | 1 | 50 |
| ΔTMD② | 32 | 3 | 5 | 5 | 1.5 | 1.5 | 1 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10sec | 25 cycles |
| 68℃ | 4min | |
| 4℃ | forever |
Add DpnI 2µL and incubate 1h.
Transformation
| Sample | Conc(/µL) | Sample Volum(µL) | Competent Cell(µL) | Total | Plate | Incubation |
|---|---|---|---|---|---|---|
| ΔTMD① | - | 4 | 50 | 54 | LB kan | 8/6~8/9 |
| ΔTMD② | - | 4 | 50 | 54 | ||
| 2-17-F | - | 2 | 50 | 52 | ||
| 2-I-5 | 2 | 50 | 52 | LB amp |
Monday, August 9
By:Wataru, Tomonori, Ken, Takuya
1. Miniprep of MS and ML
| Sample number | concentration(ng/µL) |
|---|---|
| MS | 116.2 |
| ML | 146.6 |
2. Transfotrmation of MS and ML
| Sample | conc(ng/µL) | Sample vol(µL) | Competent Cell | Competent cell vol(µL) | Total vol(µL) | Plate | Incuvation |
|---|---|---|---|---|---|---|---|
| MS | 116.2 | 2 | KRX | 50 | 52 | LB kanamycin | 8/9 18:00‾8/10 12:00 |
| ML | 146.6 | 2 | KRX | 50 | 52 |
3. Restriction enzyme digestion and ethanol precipitation
To use lac p for next ligation, we digested 1-6-G by EroRI and PstI
| Sample | 10x Buffer | BSA | Enzyme (EcoRI) | Enzyme (PstI) | MilliQ | Total |
|---|---|---|---|---|---|---|
| 50 | 6 | 0.6 | 0.5 | 0.5 | 2.4 | 60 |
Incubate 37℃ 8/9 16:20‾18:20
After restriction enzyme digestion, we did ethanol precipitation.
4. Ligation and Transformation
| Sample | Conc (nu/µL) | Sample vol (µL) | Competent cell | Competent cell vol (µL) | Total vol (µL) | Plate | Incuvation |
|---|---|---|---|---|---|---|---|
| Lac p (low) | - | 2 | KRX | 50 | 52 | LB kanamycin | 8/9 20:00‾8/10 9:00 |
| 2 | C2 | 50 | 52 |
Tuesday, August 10
By: Wataru, Tomonori, Ken, Fumitaka
Making culture and Master plate
Making culture plate on lac p (low), MS and ML
| Lac p (low) | KRX | Many colonies |
| C2 | ||
| MS | KRX | |
| ML | KRX |
Minprep of ΔTMD1+GFP
| Sample number | Concentration (ng/µL) |
|---|---|
| 1-1 | 9.9 |
| 1-2 | 27.3 |
| 2-1 | 43.2 |
| 2-2 | 34.7 |
37℃ 8/9 18:00‾8/10 9:00
Culture and Master plate
Wednesday, August 11
By: Wataru, Naoi, Ken, Takuya
| Sample | medium | Cloud | Incubation |
|---|---|---|---|
| 1 | Kanamycin | o | 37℃8/10 20:00‾8/11 9:00 |
| Ampicillin | x | ||
| 2 | Kanamycin | o | |
| Ampicillin | o | ||
| 3 | Kanamycin | o | |
| Ampicillin | x | ||
| 4 | Kanamycin | o | |
| Ampicillin | x | ||
| 5 | Kanamycin | o | |
| Ampicillin | x | ||
| 6 | Kanamycin | o | |
| Ampicillin | o | ||
| 7 | Kanamycin | o | |
| Ampicillin | x |
Discussion: About sample 1, 3, 4, 5 and 7, lac promoter was correctly inserted in low copy plasmid. About sample 2 and 6, low copy plasmid and vector derived from lac promoter were ligated. We decided to use sample 1 or 3.
Miniprep of C2+lac(low), S-R-Rz 1', 3'
lac(low)1 : 31.2 (ng/µL) lac(low)2 : 29.9 (ng/µL)
RE and electrophoresis of lac (low) 1 and 3
| Sample name | 1 | 2 | 3 | N |
| EcoRI | 0.2 | - | 0.2 | - |
| PstI | - | 0.2 | 0.2 | - |
Sample: 1-1, 1-2, 1-3, 1-N, 3-1, 3-2, 3-3, 3-N
M 1-1 1-2 1-3 1-N M M 3-1 3-2 3-3 3-N M
Discussion: Each enzyme correctly cut samples.
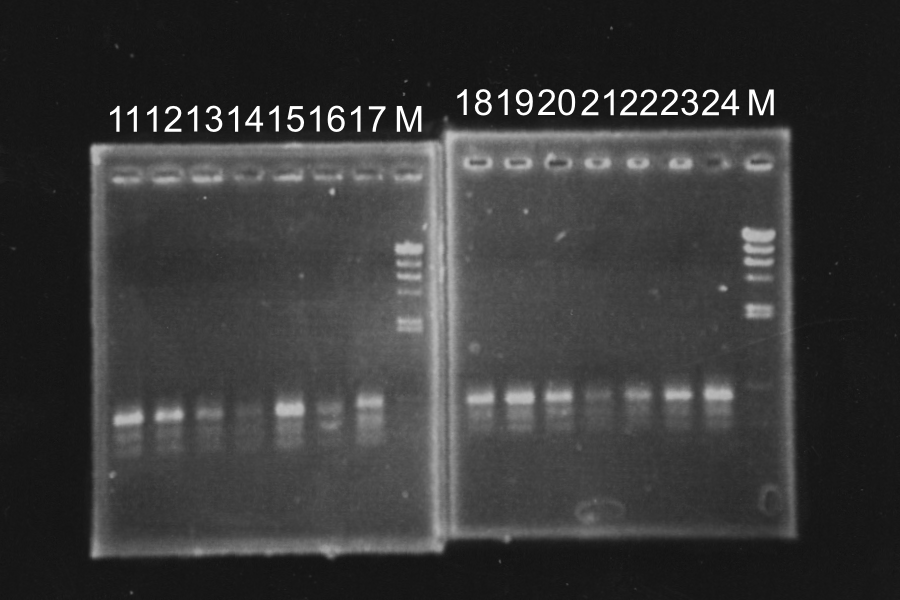
Screening PCR of SRRz
Sample: 1‾20 Control: P(1-23L) P'(2-8E) N Maker: lambda
M N P P' P 1 2 3 4 5 6 M
7 8 9 10 11 12 13 M 14 15 16 18 19 20 M
Discussion: All of the sample were self-ligation of DT and SRRz weren't inserted.
Thursday, August 12
By: Wataru, Ken
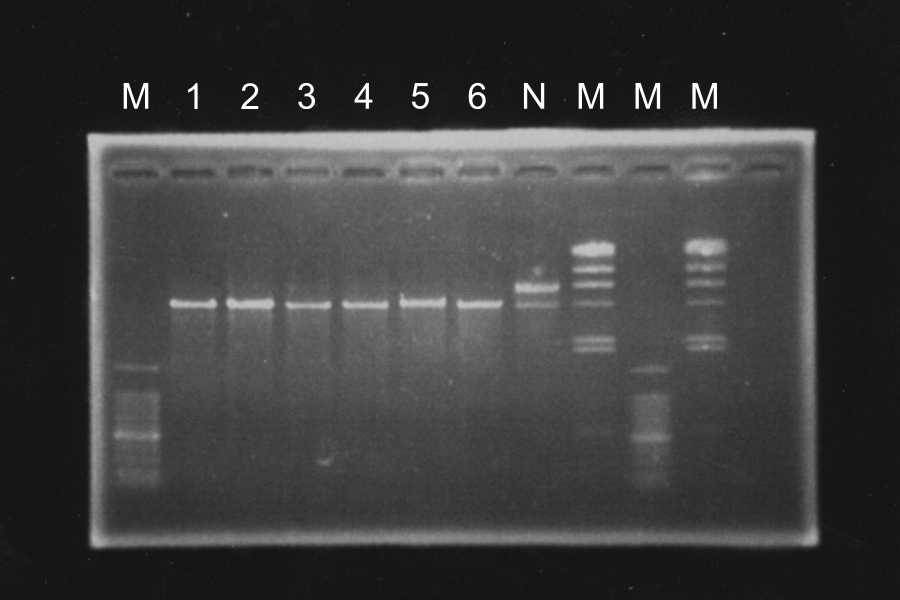
RE and electrophoresis of DT
| Sample name | Template | 10xbuffer | 100xbuffer | EcoRI | XbaI 1 | XbaI 2 | SpeI | PstI 1 | PstI 2 | Water | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 1 | 0.1 | 0.2 | - | - | - | - | - | 5.7 | 10 |
| 2 | 3 | 1 | 0.1 | - | 0.2 | - | - | - | - | 5.7 | 10 |
| 3 | 3 | 1 | 0.1 | - | - | 0.2 | - | - | - | 5.7 | 10 |
| 4 | 3 | 1 | 0.1 | - | - | - | 0.2 | - | - | 5.7 | 10 |
| 5 | 3 | 1 | 0.1 | - | - | - | - | 0.2 | - | 5.7 | 10 |
| 6 | 3 | 1 | 0.1 | - | - | - | - | - | 0.2 | 5.7 | 10 |
| N | 3 | 1 | 0.1 | - | - | - | - | - | - | 5.9 | 10 |
Sample: 1‾6, N Maker: lambda, 100
M 1 2 3 4 5 6 N M M M
Discussion: Each enzyme correctly cut each sample and was active.
Thursday, August 19
By: Wataru, Tomo, Ken
1. Miniprep of ΔTMD1GFP
29.6(ng/µg)
2. Point mutation PCR of ΔTMD1GFP
| Sample number | Template | 10xbuffer | dNTPs | MgSO4 | Primer 1 | Primer 2 | Water | KOD-plus- | Total |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.5 | 5 | 5 | 3 | 1.5 | 1.5 | 31.5 | 1 | 50 |
| 2 | 1.5 | 5 | 5 | 3 | 1.5 | 1.5 | 31.5 | 1 | 50 |
| control | 1.5 | 5 | 5 | 3 | 1.5 | 1.5 | 32.5 | - | 50 |
3. PCR condition
| 94(℃) | 2min | |
| 98 | 10sec | 30cycles |
| 55 | 30sec | |
| 68 | 3.5min | |
| 4.0 | hold |
. RE(DpnI): 17:50‾18:50
. Electrophoresis
Sample: 1, 2, Control Marker: lambda, 100
Ligation and Transformation
We named point mutation PCR products rΔTMD1GFP.
Monday, August 23
By: Wataru, Tomo, Ken, Humitaka, Tasuku
1. Miniprep of ΔTMD1
| Sample number | Concentration(ng/µg) |
| 1-1 | 58.9 |
| 2-2 | 49.9 |
2. Sequencing of ΔTMD1 and MS
Sample: rδTMD1GFP1-1, 2-2, and MS
Discussion: The sequencing was in success and the results were desirable. It meant point mutation of δTMD1GFP was succeeded and sequence of MS was confirmed. We decided to use rδTMD1GFP.
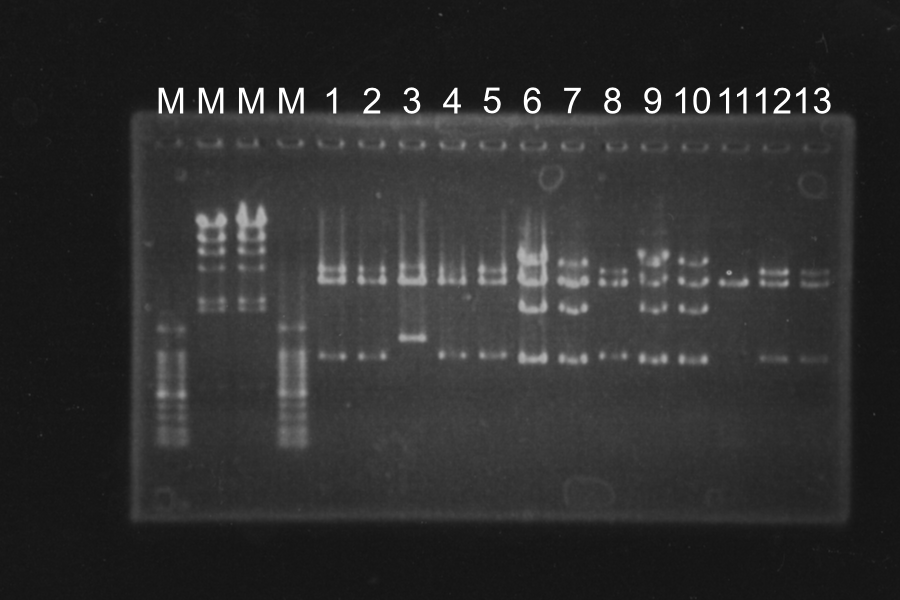
3. Screening PCR of SRRz-DT
Sample: 1‾13, Marker: lambda and 100, Control:P(1-23L) and N
PCR condition
| 90℃ | 10min | |
| 94℃ | 30sec | 35cycles |
| 50℃ | 30sec | |
| 72℃ | 1.5min | |
| 72℃ | 4min | |
| 4℃ | hold |
M 1 2 3 4 5 6 7 8 9 10 11 12 13 P N M
Discussion: We found the band; about 200bp, and it meant the lligation was completed successfully.
4. deletion PCR of rΔTMD1GFP 2-2
| Sample | 10x | dNTPs | Primer1 | Primer2 | Template | Water | KOD-plus- | Total |
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 5 | 1.5 | 1.5 | 1 | 35 | 1 | 50 |
| 2 | 5 | 5 | 1.5 | 1.5 | 1 | 35 | 1 | 50 |
| Control | 5 | 5 | 1.5 | 1.5 | 1 | 35 | - | 50 |
PCR condition
| 94℃ | 2min | |
| 94℃ | 10sec | 35cycles |
| 56℃ | 30sec | |
| 68℃ | 3.5min | |
| 4℃ | hold |
RE (DpnI) and Ligation
| Template | 25(µL) |
| DpnI | 1 |
| Total | 26 |
19:10‾20:10
| Sample Template | Water | Ligation high | T4 Kinase | total | |
|---|---|---|---|---|---|
| 1 | 3 | 6 | 5 | 1 | 15 |
| 2 | 3 | 6 | 5 | 1 | 15 |
| Control | 3 | 6 | 5 | 1 | 15 |
20:15‾21:15
Transformation We named sample 1, 2 and control rrδTMD1GFP1, 2 and control.
Tuesday, August 24
By:Ken, Tomo, Tasuku, Takuya
1.Retry of deletion PCR of rδTMD1 GFP
| Sample | 10x | dNTPs | MgSO4 | Primer1 | Primer2 | Template | Water | KOD-plus- | Total |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
| 2 | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50
Control||5||5||3||1.5||1.5||1||32||1||50 |
PCR condition
| 94℃ | 2min | |
| 94℃ | 10sec | 35cycles |
| 58℃ | 30sec | |
| 68℃ | 3.5min | |
| 4℃ | hold |
RE (DpnI), electrophoresis and ligation RE: 14:15‾15:15 Electrophoresis: Sample: 1, 2, and control, Maker: 100 and lambda M 1 2 C M
We found the band of sample 1 and 2 about 3000bp and there wasn't the band of sample control. So, we confirmed the PCR and RE were completed successfully.
2.Point mutation of SRRz
| Sample | 10x | dNTPs | MgSO4 | Primer1 | Primer2 | Template | Water | KOD-plus- | total |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
| 2 | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
| control | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
PCR condition
| 94℃ | 2min | |
| 98℃ | 10sec | 30cycles |
| 55℃ | 30sec | |
| 68℃ | 4min | |
| 4℃ | hold |
RE(DpnI), electrophoresis and ligation
We could find point mutation PCR and restriction enzyme of DpnI was done.
PCR of E0240
| Sample | 10× | dNTPs | MgSO4 | VF2 | VR | Template | Water | KOD-plus- | Total |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 31.5 | 1 | 50 |
| 2 | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 31.5 | 1 | 50 |
Purification: Sample1: 5.5*50(ng/µL) Sample2: 5.2*50(ng/µL)
RE(EcoRI, PstI) and Gel extraction Sample1: 28.8 (ng/µL) Sample2: 26.4 (ng/µL)
Transformation Sample: rrΔTMD1GFP1. 2. control, and rSRRz1. 2. control
Wednesday, August 25
By:Ken, Tomo, Kazuya, Tasuku, Takuya
Making culture and Master plate
| rrΔTMD1-1 | Many Colonies |
| rrΔTMD1-2 | |
| rrΔTMD1-C- | zero |
| rSRRz-1 | Many Colonies |
| rSRRz-2 | |
| rSRRz-C- | zero |
Miniprep of 1-5G 29.0 (ng/µL)
RE and purification of 1-5G(low copy plasmid) and lac low
| Sample name | Template | 10xbuffer | 100xbuffer | EcoRI | SpeI | PstI | Water | Total |
|---|---|---|---|---|---|---|---|---|
| 1-5G | 50 | 6 | 0.6 | 0.4 | 0.4 | - | 2.6 | 60 |
| Lac low | 10 | 4 | 0.4 | - | 0.3 | 0.3 | 25 | 40 |
| Sample Name | Concentration(ng/µL) |
| 1-5G | 18.4 |
| Lac low | 8.6 |
Ligation and transformation Ligation of E0240 and 1-5G
Thursday, August 26
By:Ken, Tomo, Kazuya, Tasuku, Takuya, Hashiya
Miniprep
| Sample name | Concentration(ng/µL) |
| constP(0.7) | 44.5 |
RE of constP(0.7)
| Template | 10xbuffer | 100xbuffer | SpeI | PstI | Water | Total |
|---|---|---|---|---|---|---|
| 25 | 4 | 0.4 | 0.3 | 0.3 | 10 | 40 |
Purification of constP (0.7) 49.8 ng/µL
Friday, August 27
By:Ken, Tomo, Kazuya, Hashiya
Making master plate of E0240 low
| Sample Name | Concentration(ng/µL) |
| rrΔTMD1 1-2 | 20.9 |
| rSRRz 1-1 | 16.4 |
RE of rrΔTMD1 and rSRRz
| Sample name | Template | 10xbuffer | 100xbuffer | XbaI | PstI | Water | Total |
|---|---|---|---|---|---|---|---|
| rrΔTMD1 1-2 | 45 | 6 | 0.6 | 0.3 | 0.3 | 7.8 | 60 |
| rSRRz 1-1 | 45 | 6 | 0.6 | 0.3 | 0.3 | 7.8 | 60 |
(13:20‾14:20)
Purification
| rrΔTMD1 1-2 | 44.7 |
| rSRRz 1-1 | 56.1 |
Lagation and transformation lacP + rrΔTMD1 1-2 constP (0.7) + rrΔTMD1 1-2 lac low + rSRRz 1-1
Monday, August 30
By: Tomonori, Kazuya, Tasuku, Ken
Making culture and Master plate
| lacP rrΔTMD1GFP | Many colonies |
| lacP rrΔTMD1GFP(control) | Some colonies |
| constP rrΔTMD1GFP | Many colonies |
| constP rrΔTMD1GFP(control) | Many colonies |
| lacP rSRRz low | No colony |
| lacP rSRRz low(control) | No colony |
Discussion: There ware some colonies, which emitted green light, on the plate 1. So, we cultured those colonies on master plate. On the plate 5 and 6, even though we used KRX, which is able to repress lac promoter, colonies might be dead. However, we still have to do some experience so that we confirm lac promoter cannot repress enough and E. coli cannot survive.
Tuesday, August 31
By: Tomonori Y, Takuya, Kazuya, Tasuku,Takuya, Ken
Miniprep
| constP (0.3) | 48.5 (ng/µL) |
| lac rrΔTMD1 | 107.3 |
RE of constP (0.3) and lac rrΔTMD1
Gel Extraction of lac rrΔTMD1
45min
Discussion: There were two band at the bottom of the gel. It was too long -45min-, and insert and vector might be contaminated. But we went on next operation.
Purification of constP (0.3) and lac rrΔTMD1
| constP (0.3) | 5.8 (ng/µL) |
| lac rrΔTMD1 | 7.8 (ng/µL) |
Ligation and transformation
| Insert | Vector |
| lac rrΔTMD1 | constP (0.3) |
Wednesday, September 1
By: Tomonori, Kazuya, Tasuku, Humitaka, Ken Making culture and Master plate
| lac rrΔTMD1 constP | many colonies |
| lac rrΔTMD1 const (control) | many colonies |
Screenig PCR of lacP-rrΔTMD1GFP-constP Sample: 1‾13 Control: Positive (1-23L) Maker: lambda, 100
M 1 2 3 4 5 6 7 8 9 10 11 12 13 P M
Discussion: All of the sample except sample 10 might be self-ligation products of constP.
Miniprep
| rSRRz 1-1 | 33.8 (ng/µL) |
| low | 56.0 (ng/µL) |
RE of rSRRz and low
| Sample name | Template | 10xbuffer | 100xbuffer | EcoRI | PstI | Water | Total |
|---|---|---|---|---|---|---|---|
| rSRRz | 20 | 4 | 0.4 | 0.3 | 0.3 | 15 | 40 |
| low | 20 | 4 | 0.4 | 0.3 | 0.3 | 15 | 40 |
(13:25‾14:30)
Purification
| rSRRz | 6.5 (ng/µL) |
| low | 16.8 |
Ligation and transformation
Insert: rSRRz 1-1 Vector: low copy plasmid
9/2
By: Tomonori, Tomo, Takuya, Ken
Making culture and Master plate
| rSRRz low | 13 colonies |
| rSRRz low (Control) | 13colonies |
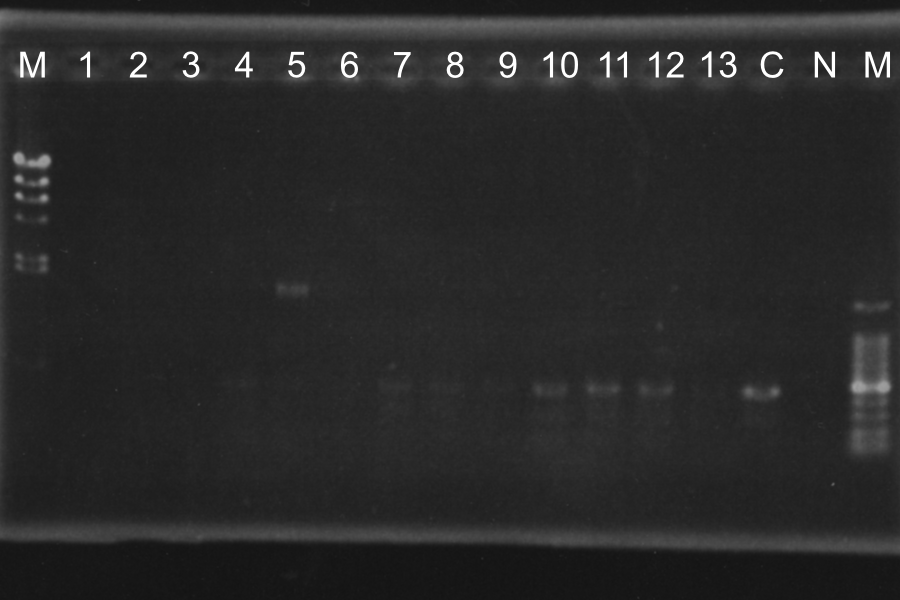
Screening PCR of rSRRz low Sample: rSRRz (1‾13) Maker: lambda, 100 Control: Positive (1-23L), Neganive
M 1 2 3 4 5 6 7 8 9 10 11 12 13 P N M
Discussion: From sample 1, two vectors might be ligated. Sample 3 and 4, rSRRz might be inserted in low copy plasmid correctly. Sample 11, it might be the self-ligation product of low copy plasmid. Anyway, we decided to culture those 4 colonies on master plate.
9/3
By: Tomonori, Tomo, Kazuya, Tasuku, Humitaka, Ken
Making culture
lac rrΔTMD1 1, 3 rrΔTMD1 1-1, 1-2 rSRRz 1-1, 1-2
ML "
"