Team:Kyoto/Notebook
From 2010.igem.org
(Difference between revisions)
(→) |
|||
| Line 8: | Line 8: | ||
|+ Tuesday, July 20 | |+ Tuesday, July 20 | ||
|- | |- | ||
| - | | | + | | By: Wataru, Tomo, Yuki, Kazuya, Ken, Makoto |
| - | + | ||
| - | + | ||
|- | |- | ||
| - | |(1) | + | | (1) Solubilization of antibiotics. |
| - | + | | For Ampicillin(Amp): add 1.0g Amp to 20ml MilliQ (Final concentration is 50mg/ml). | |
| - | + | | For Kanamycin(Kan): add 0.5g Kan to 10ml MilliQ (Final concentration is 50mg/ml). | |
| - | + | | Dispense 1.1ml of the solution into 1.5ml tubes. | |
| - | + | | Store in the freezer (-20℃). | |
| - | + | ||
|- | |- | ||
| - | |(2) | + | | (2) Making plates for LB (Amp+) and LB (Kan+). |
|- | |- | ||
| - | |(3) [[Team:Kyoto/Protocols#Transformation|Transformation]] of iGEM Parts. | + | | (3) [[Team:Kyoto/Protocols#Transformation|Transformation]] of iGEM Parts. |
{| class="experiments" | {| class="experiments" | ||
| - | !Name||Well | + | !Name||Well||Sample (µl)||Competent Cells (µl)||Total (µl)||Plate||Incubation||Result |
|- | |- | ||
|<partinfo>J23100</partinfo>||1-18-C||1||20||21||rowspan="7"|LB (Ampicillin+)||rowspan="8"|At 37℃ 7/20 20:50 - 7/21 17:00||○ | |<partinfo>J23100</partinfo>||1-18-C||1||20||21||rowspan="7"|LB (Ampicillin+)||rowspan="8"|At 37℃ 7/20 20:50 - 7/21 17:00||○ | ||
| Line 41: | Line 38: | ||
|<partinfo>B0015</partinfo>||1-23-L||1||20||21||LB (Kanamycin+)||× | |<partinfo>B0015</partinfo>||1-23-L||1||20||21||LB (Kanamycin+)||× | ||
|} | |} | ||
| - | + | | *1 "1-18-C" means well 18C in [http://partsregistry.org/Help:Spring_2010_DNA_distribution Spring 2010 DNA Distribution Kit] Plate 1. | |
| - | + | | '''Discussion''' | |
| - | + | | A vector of "pSB4K5" is Kanamycin-resistance, however, we plated it to LB plate (Ampicillin+). | |
| - | + | | And We didn't pre-culture "B0015" despite its vector is Kanamycin-resistance. | |
| - | + | | So, it was predicted that we will fail the transformation of "pSB4K5" and "B0015". | |
|} | |} | ||
---- | ---- | ||
Revision as of 16:06, 6 September 2010
Contents |
Index
Notebook
| By: Wataru, Tomo, Yuki, Kazuya, Ken, Makoto | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (1) Solubilization of antibiotics. | For Ampicillin(Amp): add 1.0g Amp to 20ml MilliQ (Final concentration is 50mg/ml). | For Kanamycin(Kan): add 0.5g Kan to 10ml MilliQ (Final concentration is 50mg/ml). | Dispense 1.1ml of the solution into 1.5ml tubes. | Store in the freezer (-20℃). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (2) Making plates for LB (Amp+) and LB (Kan+). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) Transformation of iGEM Parts.
| *1 "1-18-C" means well 18C in [http://partsregistry.org/Help:Spring_2010_DNA_distribution Spring 2010 DNA Distribution Kit] Plate 1. | Discussion | A vector of "pSB4K5" is Kanamycin-resistance, however, we plated it to LB plate (Ampicillin+). | And We didn't pre-culture "B0015" despite its vector is Kanamycin-resistance. | So, it was predicted that we will fail the transformation of "pSB4K5" and "B0015". |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (1) Cultured plates in which colonies was observed at 37℃ from 07/21 20:50 to 07/22 17:00. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (2) Made a master plate of the above plates. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) Retried Transformation of iGEM Parts.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) PCR for S-R-Rz/Rz1 and S
|
| ||||||||||||||
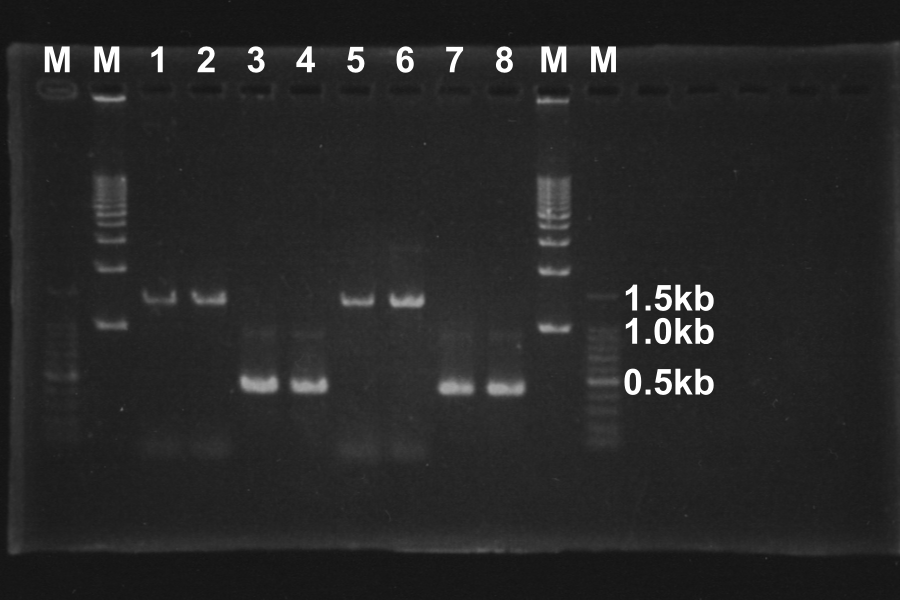
(1) Electrophoresis of the PCR products for 40min.
| ||||||||||||||
(2) Miniprep of iGEM Parts.
| ||||||||||||||
| (3) Culture plates and make master plates of <partinfo>pSB4K5</partinfo> and <partinfo>B0015</partinfo> from 7/22 17:00 to 7/23 10:00. |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(1) Miniprep of iGEM Parts.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
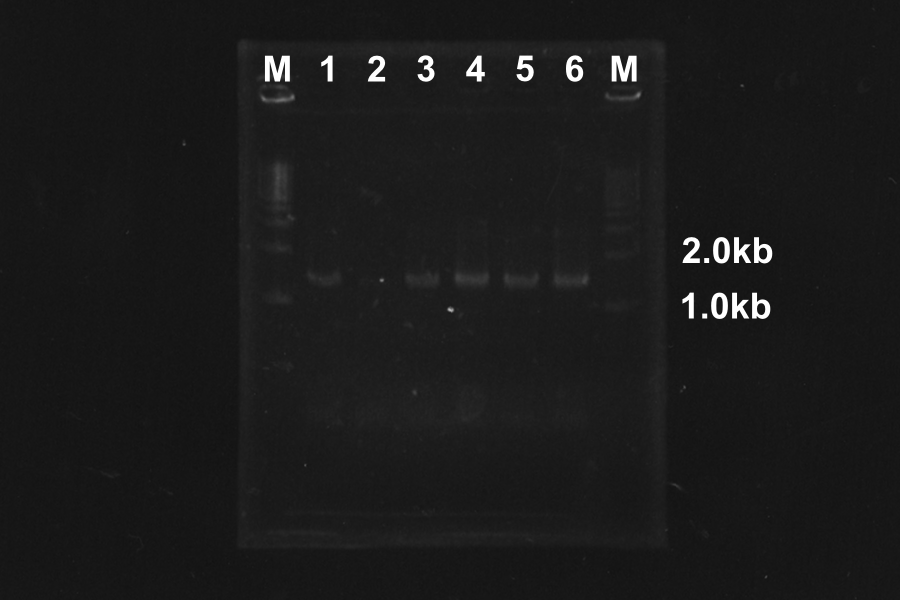
(2) Picked up 1, 3, 5, 7 of the products of PCR, and purified by PCR-purification.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) Retry of PCR of S-R-Rz/Rz1.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) Digested <partinfo>J06702</partinfo> by EcoRI, XbaI, SpeI, PstI to check function of our Restriction enzymes.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(5) Electrophoresis of above sample for 35min.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(6) To insert S gene to GFP, we digested the PCR products of S gene by EcoRi and SpeI, and GFP by EcoRl and XbaI.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ligated over night
|
| |||||||||||||||||||||||||||||
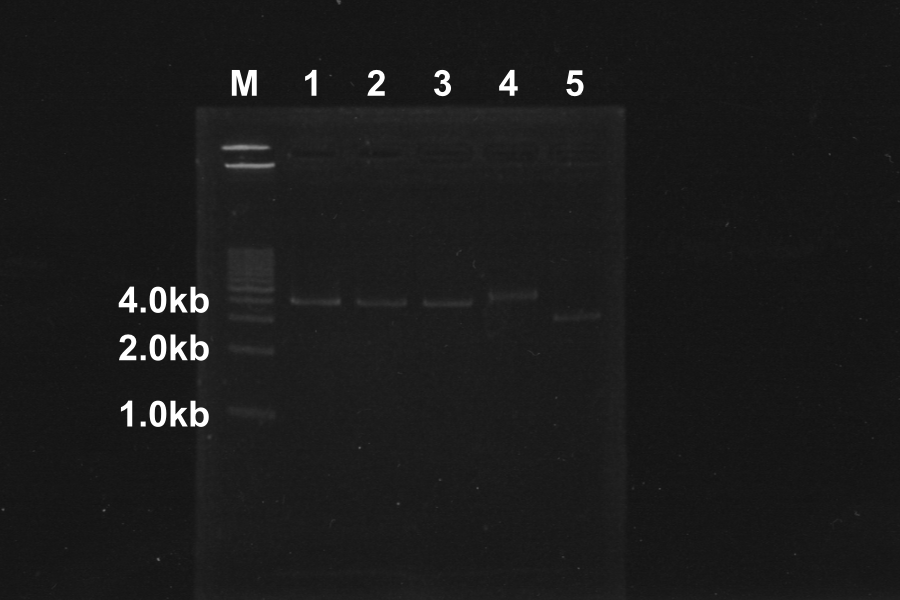
(1) Electrophoresis of PCR products
| |||||||||||||||||||||||||||||
PCR purification
| |||||||||||||||||||||||||||||
Transformation of iGEM Parts
| |||||||||||||||||||||||||||||
| Culture of 1-6-G, 1-12-O, and 1-23-L |
 "
"