September
09/09/2010
- 30s @98°C
- 1m20s @72°C
- cycle 35x
- 4°C forever
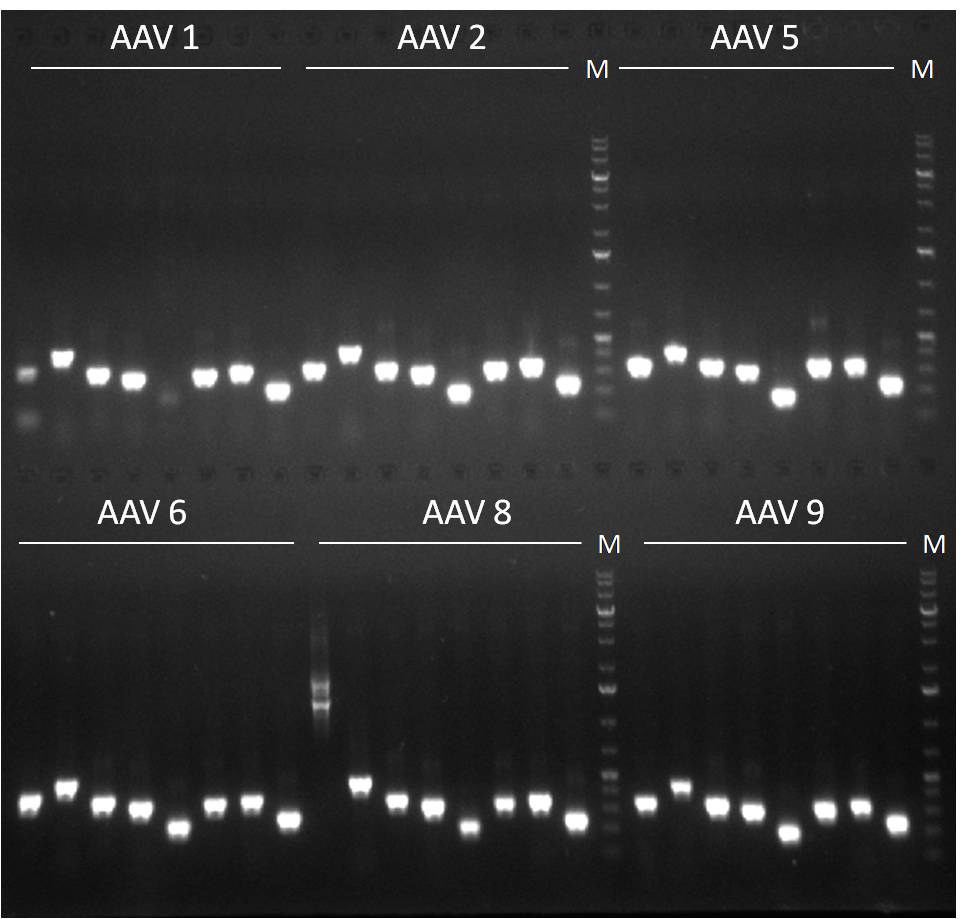
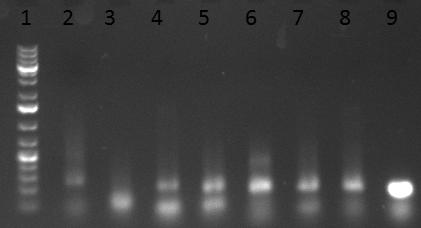
- Results of the first PCR. 8 fragments of each AAV capsid were amplified.
10/09/2010
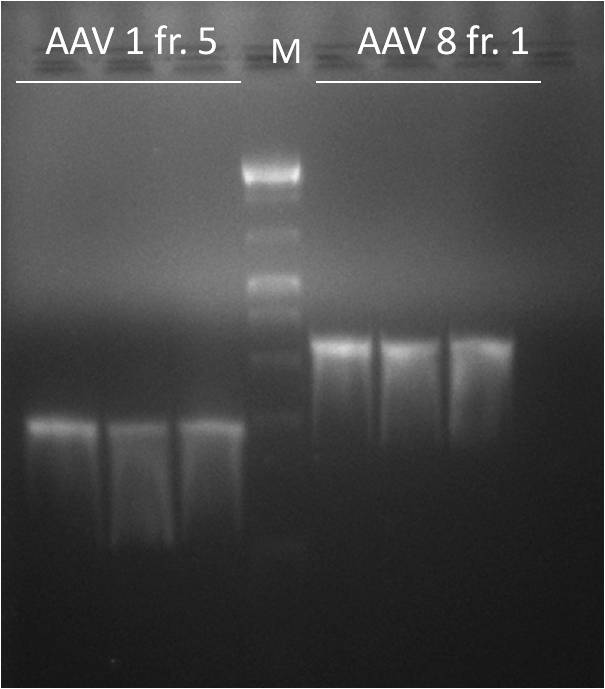
- Results of the second PCR. Fragment 5 from AAV1 as well as fragment 1 from AAV8 were amplified.
13/09/2010
- All fragments (F1,F2,F3,F4,F5,F6,F7,F8) from AAV cap1,2,5,6,8,9 were combined. Therefore 8 fragment mixes ready for restriction digest with Bsa1 were obtained. 2ul of PCR amplified fragments were taken for the restriction (NanoDrop analysis showed 1ug of DNA of each of six AAVs giving 6-7ug in total per virobyte).
- Bsa1 digestion:
- 6x2ul of DNA
- 3ul NEB4 10x buffer
- 3ul BSA 10x
- 11ul H2O
- =30ul total
- digest at 37°C for 1hr
- heat inactivation at 65°C for 20min
14/09/2010
- All fragments from previous day were washed with QIAquick nucleotide removal kit and the DNA was dissolved in 30ul H2O
- Anchor stock solution: Anchor oligos 12689 (150ul) and 5 (30ul) were added to Anchor_comp_all (180ul). The mixture was diluted 10x and then heated to 95°C for 2min and subsequently left to slowly cool down to room temperature on a heating block.
15/09/2010
- Streptavidin beads M-280, M-270, MyOne C1 and MyOne T1 activated in two replicates:
- wash twice with 75ul of wash/binding buffer and once with 75ul of 1x ligase buffer
- 20ul of Anchor to the bead solution
- incubated at RT for 1hr
- magnet applied until the solutions cleared out (M-270 is a bit slower) and the solution discarded
- wash twice with 75ul of wash/binding buffer and once with 75ul of 1x ligase buffer
- ViroByte addition started with F1, F2, F3 ,F4 and F5 following the standard protocol:
- add 20ul of Fragment X
- resuspend the beads
- add 2.3ul of 10x ligase buffer
- add 1ul of T4 ligase
- incubate at room temperature for 1hr (shake to avoid beads precipitation)
16/09/2010
- last three (F6, F7, F8) fragments added
- red replicates washed twice with 75ul W/B buffer and once with 75 ul 1x ligase buffer and diluted to 40ul water
- blue replicates washed according to same protocol and diluted into 35ul
- PCR amplification with beads:
- 25ul Phusion 2x mix
- 20ul of red replicate DNA solution
- 0.5ul of virbyte_for
- 0.5ul virbite_rev
- 4ul water
- Cycle = virbyte amp:
- 30s 94°C
- 30s 65°C
- 1m30s 72°C
- 4°C forever
- HindIII digest from beads for blue replicates:
- 35ul beads solution
- 4ul NEB2 10x
- 1ul HindIII
- incubate at 37°C for 1hr
- inactivate at 65°C for 20min
- blue replicate PCR:
- 25ul Phusion 2x mix
- 20ul of digest blue replicate DNA solution
- 0.5ul virbyte_for
- 0.5ul virbite_rev
- 4ul water
- Cycle - virbyte amp
20/09/2010
- Assembly of three fragments under diffrent conditions
- sample 1: 100ng of each fragment, gel purified
- sample 2: 100ng of each fragment, nucleotide removal purified
- sample 3: 200ng of each fragment, nucleotide removal purified
- sample 4: 100ng of each fragment, gel purified (duplicate of sample 1)
- sample 5: 300ng of each fragment, nucleotide removal purified
- PCR protocol:
- 20 cycles
- 30sec 95°C
- 30sec 60°C
- 1min 72°C (Taq Polymerase)
- 1 cyle
- 5min 72°C
- 4°C
- PCR of the samples with 6 sets of primers
- a) virobyte_fw + fragment1_cap1_rv
- b) virobyte_fw + fragment1_cap2_rv
- c) virobyte_fw + fragment1_cap5_rv
- d) virobyte_fw + fragment1_cap6_rv
- e) virobyte_fw + fragment1_cap8_rv
- f) virobyte_fw + fragment1_cap9_rv
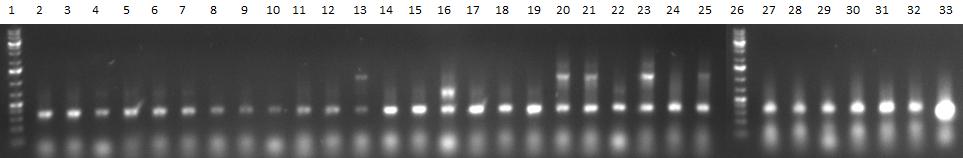
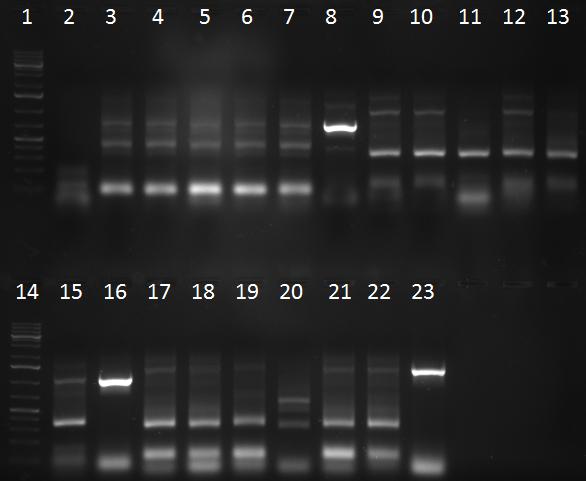
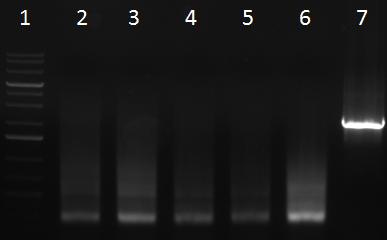
- PCR products
- 1 = 1kb ladder
- 2-7 = sample 1 with primer pairs a, b, c, d, e, f
- 8-13 = sample 2 with primer pairs a, b, c, d, e, f
- 14-19 = sample 3 with primer pairs a, b, c, d, e, f
- 20-25 = sample 4 with primer pairs a, b, c, d, e, f
- 26 = 1kb ladder
- 27-32 = sample 5 with primer pairs a, b, c, d, e, f
- 33 = positive control (fw_aav1_wildtype_fragment1 + rv_aav1_wildtype_fragment3 with aav1 cap gene)
21/09/2010
- Assembly of all eight fragments under determined conditions: 200n of DNA and 15min ligation
- PCR protocol
- 1 cycle
- 5min 95°C
- 25 cycles
- 30sec 95°C
- 30sec 60°C
- 1min 45sec 72°C (Phusion Polymerase)
- 1 cyle
- 5min 72°C
- 4°C
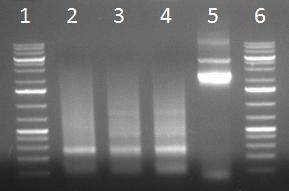
- PCR products
- 1 = 1kb ladder
- 2 = 8 fragment assembly
- 3 = duplication
- 4 = triplicate
22/09/2010
- 8 fragment ligation under different conditions
- sample 1: repeat of the last assembly with 200ng of each fragment and 15min ligation time
- sample 2: sinking DNA concentration from 200ng on.
- 200ng of DNA are added to the ligation reaction for the fragment 1 to 3
- 150ng of DNA are added to the ligation reaction for the fragment 4 and 5
- 100ng of DNA are added to the ligation reaction for the fragment 6 and 7
- 80ng of DNA are added to the ligation reaction for the fragment 8
- sample 3: sinking concentration
- 300ng of DNA of DNA are added to the ligation reaction for the fragment 1 to 3
- 250ng of DNA are added to the ligation reaction for the fragment 4 and 5
- 150ng of DNA are added to the ligation reaction for the fragment 6 and 7
- 100ng of DNA are added to the ligation reaction for the fragment 8
- PCR protocol
- 1 cycle
- 5min 95°C
- 25 cycles
- 30sec 95°C
- 30sec 60°C
- 1min 45sec 72°C (Phusion Polymerase)
- 1 cyle
- 5min 72°C
- 4°C
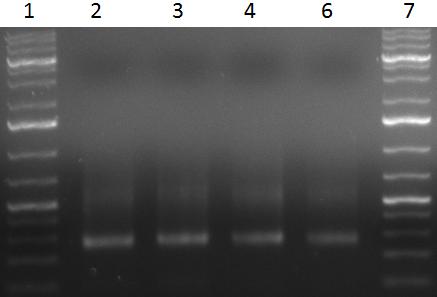
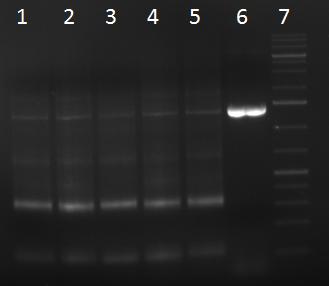
- PCR products
- 1 = 1kb ladder
- 2 = PCR repeat of sample from 21/09/10
- 3 = sample 1
- 4 = sample 2
- 5 = sample 3
- 6 = sample 1 duplicate
- 7 = sample 2 duplicate
- 8 = sample 3 duplicate
- 9 = positive control: amplification of fragment 1 from AAV1 cap gene
23/09/2010
- step to step assembly
- sample 1: fragment 1 and 2 are assembled to the anker
- sample 2: fragment 1, 2 and 3 are assembled t the anker
- sample 3: fragment 1 to 4 are assembled to the anker
- 200ng of each fragment are used for every sample and 10min of ligation time
- PCR protocol
- 1 cycle
- 5min 95°C
- 25 cycles
- 30sec 95°C
- 30sec 60°C
- 45sec 72°C (Phusion Polymerase)
- 1 cyle
- 5min 72°C
- 4°C
- 1 = 1kb ladder
- 2 = sample 1 pcr amplified with virobyte_fw primer and F2_cap1_rv primer
- 3 = sample 1 pcr amplified with virobyte_fw primer and F2_cap2_rv primer
- 4 = sample 1 pcr amplified with virobyte_fw primer and F2_cap5_rv primer
- 5 = sample 1 pcr amplified with virobyte_fw primer and F2_cap6_rv primer
- 6 = sample 1 pcr amplified with virobyte_fw primer and F2_cap8_rv primer
- 7 = sample 1 pcr amplified with virobyte_fw primer and F2_cap9_rv primer
- 8 = control of sample 1: fragment 1 and 2 amplified from AAV1 cap gene
- 9 = sample 2 pcr amplified with virobyte_fw primer and F3_cap1_rv primer
- 10 = sample 2 pcr amplified with virobyte_fw primer and F3_cap2_rv primer
- 11 = sample 2 pcr amplified with virobyte_fw primer and F3_cap5_rv primer
- 12 = sample 2 pcr amplified with virobyte_fw primer and F3_cap6_rv primer
- 13 = sample 2 pcr amplified with virobyte_fw primer and F3_cap8_rv primer
- 14 = sample 2 pcr amplified with virobyte_fw primer and F3_cap9_rv primer
- 15 = control of sample 2: fragment 1, 2 and 3 amplified from AAV1 cap gene
- 16 = sample 3 pcr amplified with virobyte_fw primer and F4_cap1_rv primer
- 17 = sample 3 pcr amplified with virobyte_fw primer and F4_cap2_rv primer
- 18 = sample 3 pcr amplified with virobyte_fw primer and F4_cap5_rv primer
- 19 = sample 3 pcr amplified with virobyte_fw primer and F4_cap6_rv primer
- 20 = sample 3 pcr amplified with virobyte_fw primer and F4_cap8_rv primer
- 21 = sample 3 pcr amplified with virobyte_fw primer and F4_cap9_rv primer
- 22 = control of sample 3: fragment 1 to 4 amplified from AAV1 cap gene
24/09/2010
- 8 fragment assembly
- 200ng of each fragment are used for every sample and 10min of ligation time
- PCR protocol
- 1 cycle
- 5min 95°C
- 25 cycles
- 30sec 95°C
- 30sec 60°C
- 1min 45sec 72°C (Phusion Polymerase)
- 1 cyle
- 5min 72°C
- 4°C
- 1 = PCR product of the 8 fragment assembly
- 2 = PCR product of control: AAV1 cap gene amplified from fragment 1 to 8
- 3 = 1kb ladder (the ladder is not seperated properly in the higher range due to the short time of running)
25/09/2010
- After gel purification the fragments were amplified again
- PCR protocol
- 1 cycle
- 5min 95°C
- 25 cycles
- 30sec 95°C
- 30sec 60°C
- 1min 45sec 72°C (Phusion Polymerase)
- 1 cyle
- 5min 72°C
- 4°C
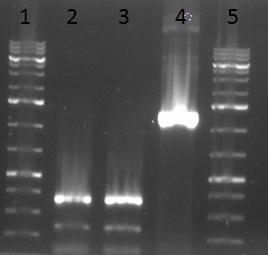
- 1 = 1kb ladder
- 2 = PCR product of purified assembly from fragment 1 to 4 amplified with virobyte_fw primer and F4_cap1_rv primer
- 3 = PCR product of purified assembly from fragment 1 to 4 amplified with virobyte_fw primer and F4_cap2_rv primer
- 4 = positive control: fragment 4 amplified from AAV1 cap gene
- 5 = 1kb ladder
- 1 = 1kb ladder
- 2 = PCR product of purified assembly from fragment 1 to 8 amplified with virobyte_fw primer and virobyte_rv primer
- 3 = duplicate
- 4 = triplicate
- 5 = PCR product (from another pcr) of purified assembly from fragment 1 to 8 amplified with virobyte_fw primer and virobyte_rv primer
- 6 = duplicate
- 7 = positive control: cap gene (fragment 1 to 8) from AAV1
27/09/2010
- amplification of the assembled 8 fragment, which where gel purified
- PCR protocol
- 1 cycle
- 5min 95°C
- 25 cycles
- 30sec 95°C
- 30sec 60°C
- 1min 45sec 72°C (Phusion Polymerase)
- 1 cyle
- 5min 72°C
- 4°C
- PCR products
- 1 = 1kb ladder
- 2 = PCR product of the 8 fragment amplification, which where gel purified. 4µl instead of 2µl are used for the PCR.
- 3 = duplicate
- 4 = PCR product (of another PCR) of the 8 fragment amplification, which where gel purified. 4µl instead of 2µl are used for the PCR.
- 5 = positive control: pcr product of AAV1 cap gene from fragment 1 to 8
- 6 = 1kb ladder
28/09/2010
- repeat of the 4 fragment assembly
- PCR protocol
- 1 cycle
- 5min 95°C
- 25 cycles
- 30sec 95°C
- 30sec 60°C
- 45sec 72°C (Phusion Polymerase)
- 1 cyle
- 5min 72°C
- 4°C
- 1 = 1kb ladder
- 2 = 4 fragment assembly pcr amplified with virobyte_fw primer and F4_cap2_rv primer
- 3 = 4 fragment assembly pcr amplified with virobyte_fw primer and F4_cap5_rv primer
- 4 = 4 fragment assembly pcr amplified with virobyte_fw primer and F4_cap6_rv primer
- 5 = positive control: fragment 1 to 4 amplified from AAV1 cap gene**6 =
- 6 = 1kb ladder
29/09/2010
- repeat of the 4 fragment assembly
- PCR protocol
- 1 cycle
- 5min 95°C
- 25 cycles
- 30sec 95°C
- 30sec 51°C
- 45sec 72°C (Phusion Polymerase)
- 1 cyle
- 5min 72°C
- 4°C
- The annealing temperature was dropped to 51°C
- 1 = 4 fragment assembly pcr amplified with virobyte_fw primer and F4_cap1_rv primer
- 2 = 4 fragment assembly pcr amplified with virobyte_fw primer and F4_cap2_rv primer
- 3 = 4 fragment assembly pcr amplified with virobyte_fw primer and F4_cap5_rv primer
- 4 = 4 fragment assembly pcr amplified with virobyte_fw primer and F4_cap6_rv primer
- 5 = 4 fragment assembly pcr amplified with virobyte_fw primer and F4_cap8_rv primer
- 6 = positive control: fragment 1 to 4 amplified from AAV1 cap gene
- 7 = 1kb ladder
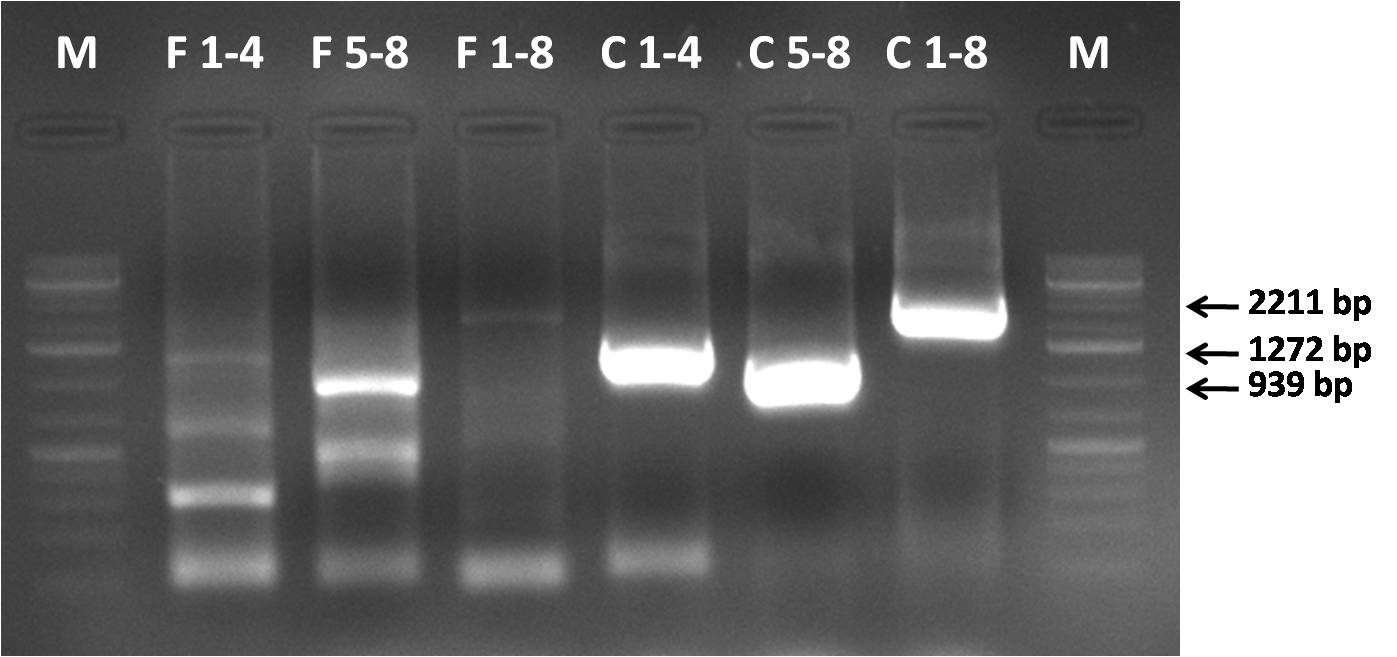
27/09/2010
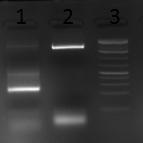
AAV 1 fragments 1-4; 5-8 and 1-8 (full capsid) assembly
- Gel loading scheme:
- M - GeneRuler 1kb DNA ladder
- F 1-4 - fragments 1-4 assembly
- F 5-8 - fragments 5-8 assembly
- F 1-8 - fragments 1-8 assembly
- C 1-4 - fragments 1-4 control
- C 5-8 - fragments 5-8 control
- C 1-8 - fragments 1-8 control
|

 "
"