Team:Groningen/Hydrophobins
From 2010.igem.org
Hydrophobins
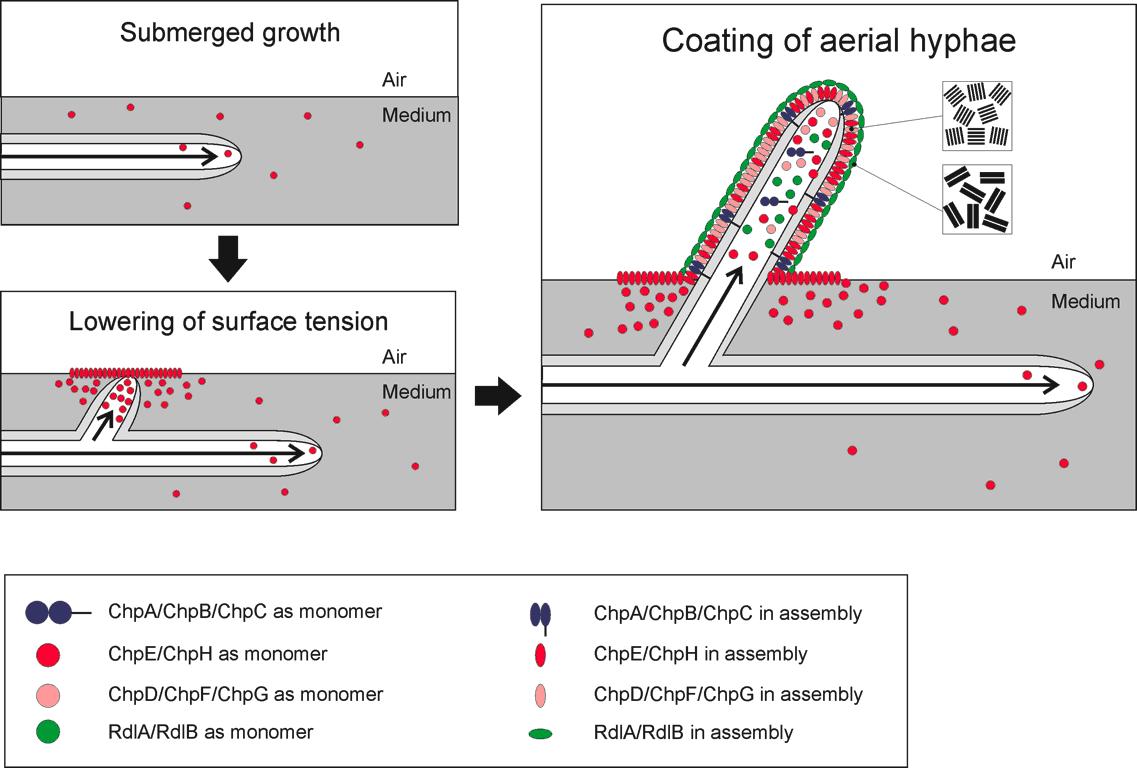
Biology The hydrophobic proteins that we are expressing in our bacillus biofilm are called chaplins and are derived from streptomyces. We streptomycyes is sporulating hyfe are trying to grow into the are. Since these hyfe are very small it is very hard to break the water air barrier for these hyfe, this is why streptomyces produces small hydrophobic proteins on the surface of it hyfe.
Three subgroups
In total their are eight different chaplins. These eight chaplins can be devided in to three groups.
Chaplin A-C This group of chaplin are the largest and are almost three times these size of the other chaplins ones around ... kD. What makes these chaplins special besides their size is that they have a cell wall anchor and a hydrophilic region as well as a hydrophobic region.
Chaplin D, F-H These chaplin are small and have only a hydrophobic region
Chaplin E This chaplin is also small but is in the vivo relaesed outside the cell to start the watertension lowering before the hyfe actually penetrates the water-air barrier
Physical properties What makes these proteins inteesting is that they are amfipathic, meening that they "in theory" can change hydrophilic surfaces into hydrophobic surfaces but in turn can also change hydrophobic surfaces into hydrophilic surfaces. Chaplin are functional amyloids that will asemble by a catalytic process from monomers in polymerics chain forming rod like structure surfaces called amyloid fibers. These fiber are very rigid and and hard to break down. These fiber can only be broken up by boiling them in SDS. share distinguishing features with the medically important pathogenic amyloid fibers that are the hallmark of many neurodegenerative diseases such as Alzheimer's, Huntington's, systemic amyloidosis and the prion diseases.
 "
"