Team:ETHZ Basel/Modeling
From 2010.igem.org
Mathematical Modeling Overview
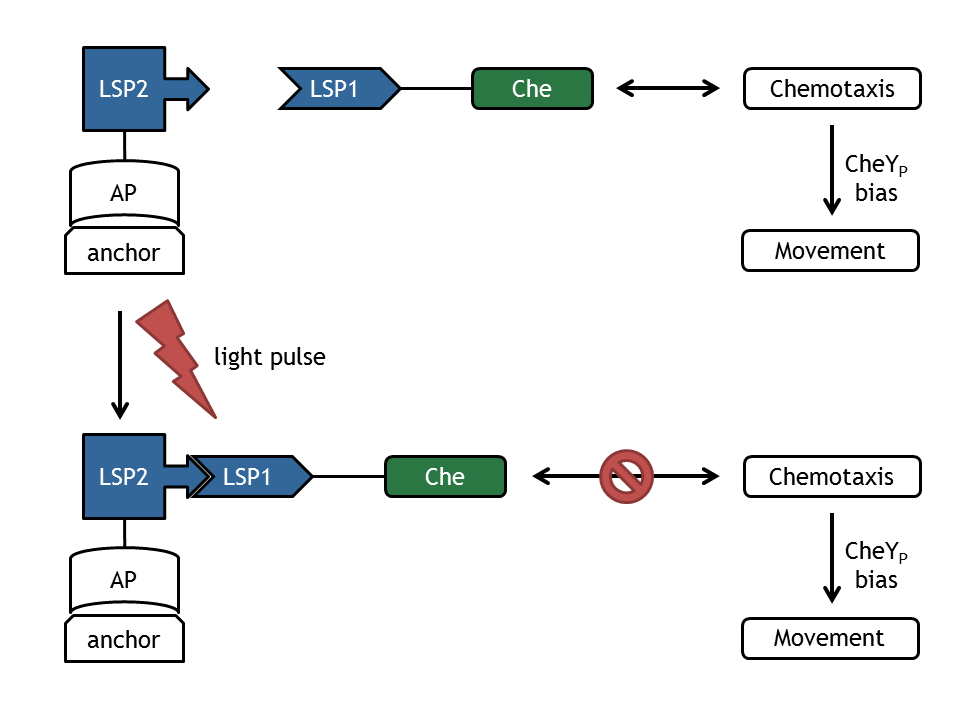
In order to support wet laboratory experiments and to create a test bench for the information processing part, a complex mathematical model of E. lemming was created. This goal was achieved by implementing and combining deterministic molecular models of the chemotaxis pathway and the light switch and probabilistic model for the bacterial movement.
Model implementation
Individual molecular models
In a first step, we implemented individual deterministic molecular models for the subdevices and a stochastic mathematical model of the bacterial movement.
- Chemotaxis Pathway: two similar models of the chemotactic receptor pathway.
- Light Switch: based on the light-sensitive dimerizing Arabidopsis proteins PhyB and PIF3.
- Bacterial Movement: a probabilistic model of E. coli movement, determined by distribution of input bias.
Combined mathematical models
The next step, combining the individual models to a comprehensive and more complex model of E. lemming was achieved in two substeps:
- Light switch - Chemotaxis: used to provide support for wet laboratory.
- Chemotaxis - Movement: complete model of E. lemming.
Experimental Design
Insights for wet laboratory
Since the design of E.lemming implies the existence of many possible biological combinations of the fundamental parts, the first steps in the modeling of our system were directed towards reducing the actual number of combinations implemented in the wetlab. Furthermore, by using the combined molecular models for in silico evaluation of the best possible devices, we derived theoretical results on choosing the biological parts which maximize the chance of a functional final ensemble.
Wet laboratory evaluation results have showed that molecular modeling and experimental biology can interwork to gain new insight on both aspects of our project.
Insights for information processing
In order to adjust the controller to have optimal light pulse rates, the combined molecular model has been used to determine the corresponding time constants.
Information processing evaluation results provide further information how this has been accomplished.
Test bench for information processing
In order to create a first test bench for the information processing pipeline, the combined model has been used to set up and evaluate the controller. By providing input ports for the actual and the desired movement direction of the bacterium and boolean output ports for both light pulses (red light/far red light), it was possible to close the loop and simulate the entire system.
 "
"




