Team:Chiba/System 2
From 2010.igem.org
(→Overall Circuit) |
(→Testing) |
||
| (30 intermediate revisions not shown) | |||
| Line 248: | Line 248: | ||
=Version 2= | =Version 2= | ||
| - | == | + | ==Overview== |
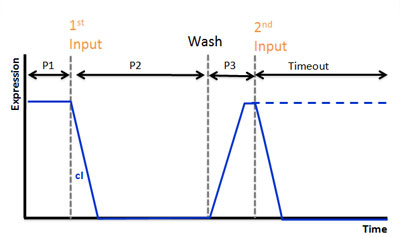
| - | Our second circiut of double click system is called <b>Recognize circuit</b>.<br> | + | {|- |
| + | |Our second circiut of double click system is called <b>Recognize circuit</b>.<br> | ||
It consists of <b>recognizing</b> system and <b>memorizing</b> system.<br> | It consists of <b>recognizing</b> system and <b>memorizing</b> system.<br> | ||
Recognizing system can recognize the existence of input.<br> | Recognizing system can recognize the existence of input.<br> | ||
And Memorizing system can memorize the existence of input in the past for a while.<br> | And Memorizing system can memorize the existence of input in the past for a while.<br> | ||
When the two system work together, bacteria is going to shine.<br> | When the two system work together, bacteria is going to shine.<br> | ||
| - | We hope this circuit works as double click system. | + | We hope this circuit works as double click system. |
| + | |[[Image:Chiba_cI.jpg]] | ||
| + | |} | ||
We use AHL for input and regard injection of AHL as clicking. In our project, CLICK controls transcription of overall DNA sequence. <br> | We use AHL for input and regard injection of AHL as clicking. In our project, CLICK controls transcription of overall DNA sequence. <br> | ||
| - | In recognizing input system, there is a cI operator above gfp DNA sequence.cI recognizes the existence of input in this DNA sequence.<br> | + | In recognizing input system, there is a cI operator above gfp DNA sequence. cI recognizes the existence of input in this DNA sequence.<br> |
In memorizing system, there is an AND Gate with T7ptag and supD. In this case, the AND Gate remembers that there was a click.<br> | In memorizing system, there is an AND Gate with T7ptag and supD. In this case, the AND Gate remembers that there was a click.<br> | ||
There is a hybrid promoter(cI/T7) above gfp DNA sequence. It is regulated by T7 and cI which work as activator and repressor, respectively. On this promoter, repression is stronger than activation. And also, it is low unregulated activation. Working of this promoter depends on recognizing and memorizing system.<br> | There is a hybrid promoter(cI/T7) above gfp DNA sequence. It is regulated by T7 and cI which work as activator and repressor, respectively. On this promoter, repression is stronger than activation. And also, it is low unregulated activation. Working of this promoter depends on recognizing and memorizing system.<br> | ||
| - | [Team:Chiba/ | + | [[Team:Chiba/System_2/Overall|details...]] |
<br><br><br> | <br><br><br> | ||
| - | == | + | ==Circuit Construction== |
| - | + | ||
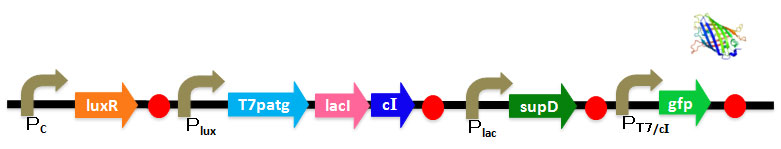
We've designed DNA sequence like following Figure. <br> | We've designed DNA sequence like following Figure. <br> | ||
<center> | <center> | ||
[[Image:Chiba_Sys2.jpg]]</center> | [[Image:Chiba_Sys2.jpg]]</center> | ||
| - | Based on this sequence, we've also designed | + | Based on this sequence, we've also designed vector.<br> |
<center> | <center> | ||
[[Image:Chiba_ver2.jpg]]</center> | [[Image:Chiba_ver2.jpg]]</center> | ||
| - | Prom-luxR&PT7/cI-GFP vector is constructed collaboration with | + | Prom-luxR&PT7/cI-GFP vector is constructed collaboration with Double Click System Version 1.<br><br><br> |
| + | ==Result== | ||
===Testing=== | ===Testing=== | ||
In this system, lux promoter inverter is sensor of input. | In this system, lux promoter inverter is sensor of input. | ||
We've researched Plux inverter and got some results.<br> | We've researched Plux inverter and got some results.<br> | ||
| + | We need lux promoter repressed by AHL-luxR dimer. But Plux inverter of biobrick didn't work. So we've tried to charaterize it and make Plux inverter.<br> | ||
| + | LuxR is AHL-dependent activator. LuxR-AHL complex binds lux box, 20-bp sequence centered at position -42.5 from starting site and activates transcription. However lux box is inserted between -35 and -10,LuxR functions as AHL-inverter (Plux inv). Plux inv is resistered in Biobrick number R0061. We've prepared Plux inv-GFP and characterized about it. [[Team:Chiba/System_2/Result|details...]] | ||
| + | |||
===Evaluation=== | ===Evaluation=== | ||
<br> | <br> | ||
| Line 283: | Line 289: | ||
==Conclusion== | ==Conclusion== | ||
| - | + | It does not completely repress transcription, Plasmids, strains ,culture conditions,and detection method LuxR suppression can not be confirmed(Cox et al,2007).<br> | |
| - | + | [[Team:Chiba/System_2/Result|details...]] | |
| - | + | <br><br><br> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | It | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Latest revision as of 03:34, 28 October 2010

Contents |
Version 2
Overview
We use AHL for input and regard injection of AHL as clicking. In our project, CLICK controls transcription of overall DNA sequence.
In recognizing input system, there is a cI operator above gfp DNA sequence. cI recognizes the existence of input in this DNA sequence.
In memorizing system, there is an AND Gate with T7ptag and supD. In this case, the AND Gate remembers that there was a click.
There is a hybrid promoter(cI/T7) above gfp DNA sequence. It is regulated by T7 and cI which work as activator and repressor, respectively. On this promoter, repression is stronger than activation. And also, it is low unregulated activation. Working of this promoter depends on recognizing and memorizing system.
details...
Circuit Construction
We've designed DNA sequence like following Figure.
Based on this sequence, we've also designed vector.
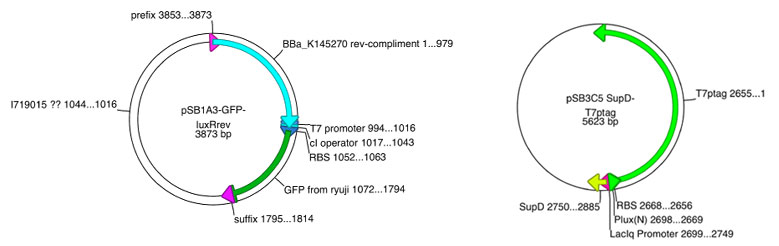
Prom-luxR&PT7/cI-GFP vector is constructed collaboration with Double Click System Version 1.
Result
Testing
In this system, lux promoter inverter is sensor of input.
We've researched Plux inverter and got some results.
We need lux promoter repressed by AHL-luxR dimer. But Plux inverter of biobrick didn't work. So we've tried to charaterize it and make Plux inverter.
LuxR is AHL-dependent activator. LuxR-AHL complex binds lux box, 20-bp sequence centered at position -42.5 from starting site and activates transcription. However lux box is inserted between -35 and -10,LuxR functions as AHL-inverter (Plux inv). Plux inv is resistered in Biobrick number R0061. We've prepared Plux inv-GFP and characterized about it. details...
Evaluation
Remark&Notes
Conclusion
It does not completely repress transcription, Plasmids, strains ,culture conditions,and detection method LuxR suppression can not be confirmed(Cox et al,2007).
details...
 "
"