Team:Cambridge/Bioluminescence/Background Firefly
From 2010.igem.org
(→The Light Emitting Reaction) |
|||
| Line 1: | Line 1: | ||
{{:Team:Cambridge/Templates/headerMinimalprototype}} | {{:Team:Cambridge/Templates/headerMinimalprototype}} | ||
{{:Team:Cambridge/Templates/headerbar|colour=#96d446|linkcolour=#6bbe00|title=Project Firefly: Background}} | {{:Team:Cambridge/Templates/headerbar|colour=#96d446|linkcolour=#6bbe00|title=Project Firefly: Background}} | ||
| - | =The Light Emitting Reaction | + | {{:Team:Cambridge/Templates/Topheader|header=The Light-Emitting Reaction}} |
{{:Team:Cambridge/Templates/RightImage|image=Firefly_display.jpg|caption=Luciola mating display}} | {{:Team:Cambridge/Templates/RightImage|image=Firefly_display.jpg|caption=Luciola mating display}} | ||
The design of our BioBricks was based on an understanding of the bioluminescence reactions as displayed in the diagram below. D-luciferin is converted into oxyluciferin by the enzyme luciferase using ATP and oxygen as well as magnesium as a co-factor. Oxyluciferin is generated in an unstable, energetically excited state, but rapidly returns to the more stable ground state. This is lower in energy and the energy released during the process takes the form of an emitted photon of visible light. The generation of light in this reaction is extremely efficient as much of the energy released by the chemical reaction is converted into light, with hardly any loss to heat production ([http://www.nature.com/nphoton/journal/v2/n1/full/nphoton.2007.259.html Ugarova 2008]). | The design of our BioBricks was based on an understanding of the bioluminescence reactions as displayed in the diagram below. D-luciferin is converted into oxyluciferin by the enzyme luciferase using ATP and oxygen as well as magnesium as a co-factor. Oxyluciferin is generated in an unstable, energetically excited state, but rapidly returns to the more stable ground state. This is lower in energy and the energy released during the process takes the form of an emitted photon of visible light. The generation of light in this reaction is extremely efficient as much of the energy released by the chemical reaction is converted into light, with hardly any loss to heat production ([http://www.nature.com/nphoton/journal/v2/n1/full/nphoton.2007.259.html Ugarova 2008]). | ||
Latest revision as of 22:57, 27 October 2010

The Light-Emitting Reaction
The design of our BioBricks was based on an understanding of the bioluminescence reactions as displayed in the diagram below. D-luciferin is converted into oxyluciferin by the enzyme luciferase using ATP and oxygen as well as magnesium as a co-factor. Oxyluciferin is generated in an unstable, energetically excited state, but rapidly returns to the more stable ground state. This is lower in energy and the energy released during the process takes the form of an emitted photon of visible light. The generation of light in this reaction is extremely efficient as much of the energy released by the chemical reaction is converted into light, with hardly any loss to heat production (Ugarova 2008).
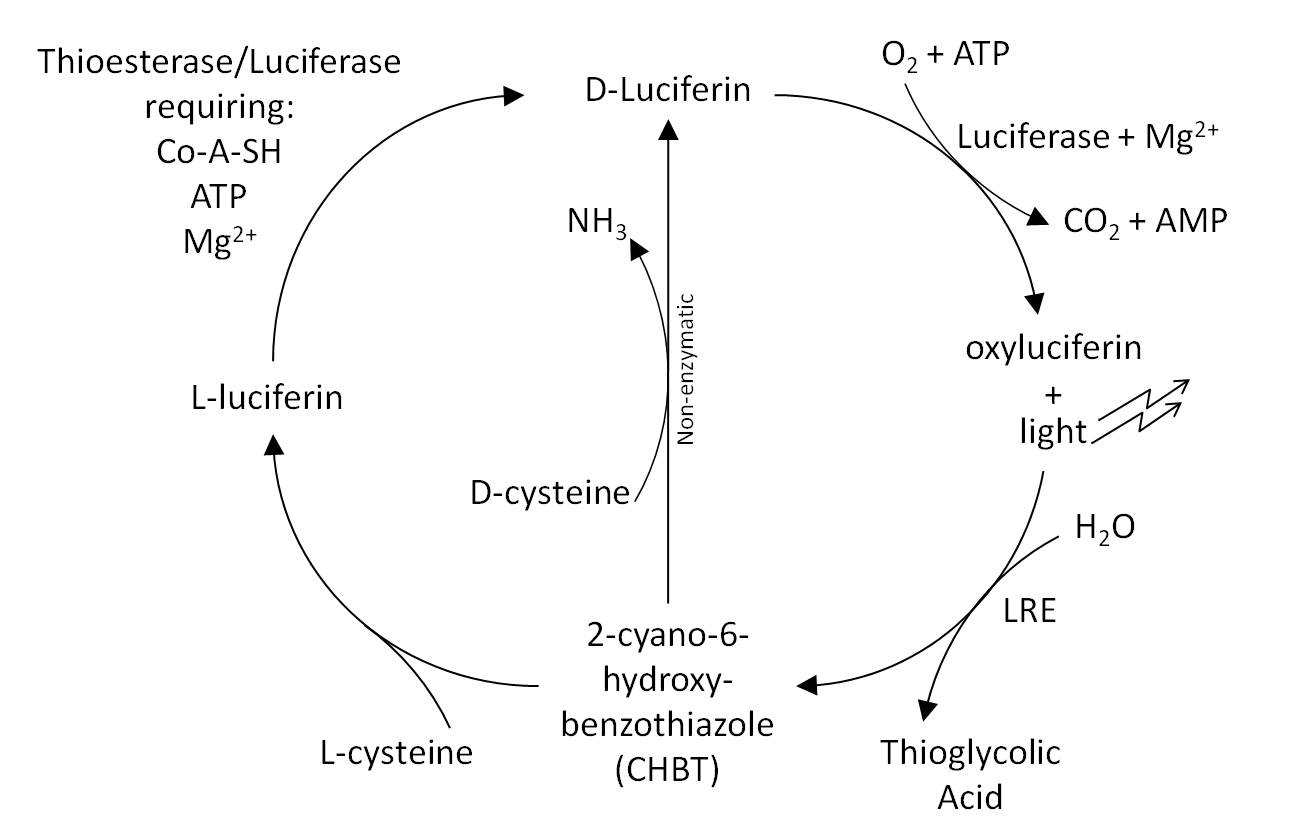
The Luciferin Cycle
The luciferin-regenerating enzyme (LRE) then converts oxyluciferin into 2-cyano-6-hydroxybenzothiazole (CHBT), which is non-enzymatically converted back into D-luciferin using D-cysteine (Gomi and Kajiyama 2001). An alternative route has been reported in which D-luciferin is regenerated via L-luciferin. This pathway requires L-cysteine and invovles the enzymes thioesterase and luciferase together with Coenzyme A, Magnesium and ATP.
Insect luciferases are often used as reporter genes to study biochemical, physiological and pathological processes. Despite great research interest, the biosynthetic pathway leading to the de-novo production of D-luciferin and the genes responsible have not been identified yet.
 "
"