Team:Calgary/20 July 2010
From 2010.igem.org
Raidakhwaja (Talk | contribs) |
|||
| Line 3: | Line 3: | ||
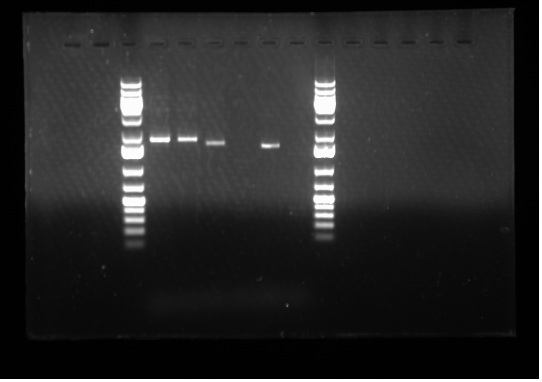
[[Image:07.20.2010. Raida PCR BBK primer.jpg|thumb|400px|Gel electrophoresis of Raida's PCR products: BBK primers that anneal before the multiple cloning sites. Lane 4 and 5 : Lux0047 A + B0015 psB1AK3 Lane 6: J23002 + Lux0047E, Lane7: R0040 + I3502, Lane 8: Lux0047E, Lane 9: Master Mix]] | [[Image:07.20.2010. Raida PCR BBK primer.jpg|thumb|400px|Gel electrophoresis of Raida's PCR products: BBK primers that anneal before the multiple cloning sites. Lane 4 and 5 : Lux0047 A + B0015 psB1AK3 Lane 6: J23002 + Lux0047E, Lane7: R0040 + I3502, Lane 8: Lux0047E, Lane 9: Master Mix]] | ||
| - | |||
<u> Raida </u> | <u> Raida </u> | ||
| Line 15: | Line 14: | ||
Today I completed the ligation and transformation of the I0500+E0034 plasmid switch. I also ran the PCR products and the results came back negative. So I set up another PCR and Gel. | Today I completed the ligation and transformation of the I0500+E0034 plasmid switch. I also ran the PCR products and the results came back negative. So I set up another PCR and Gel. | ||
| - | |||
<u>Patrick</u> | <u>Patrick</u> | ||
| Line 24: | Line 22: | ||
Today I plated the construction that I did last night. I also overnight cultured the construction from last night by inoculating from the cells to be plated. Tomorrow I will plasmid prep the cultures. I also looked into the literature regarding the plasmid which will be presented to Paul on Thursday. | Today I plated the construction that I did last night. I also overnight cultured the construction from last night by inoculating from the cells to be plated. Tomorrow I will plasmid prep the cultures. I also looked into the literature regarding the plasmid which will be presented to Paul on Thursday. | ||
| + | |||
| + | <u>Chris</u> | ||
| + | |||
| + | Today, I did a colony PCR of the CpxP promoter that was transformed into both ampicillin-kanamycin and ampicillin-chloramphenicol plasmids. The results of the colony PCR were then run on a 1.2% gel and the bands were very irregular as shown on the right. The gel was run for approximately 1 hour and the bands did not move after 35 minutes. Another colony PCR was set up and let to run again overnight. As well, overnight culures were made of all 11 samples so they could be plasmid prepped appropriately if the Colony PCR showed up possible correct bands. As well, I helped Emily test the plate reader with several cultures that had arabinose inducible promoters induced overnight of GFP and RFP. | ||
| + | |||
}} | }} | ||
Revision as of 02:08, 21 July 2010

Tuesday July 20, 2010
Raida
Today I set up and ran two 1% gels: one for my PCR products from yesterday and the second one for Emily's PCR products- both of which were done to test whether our primers are working or not. Please see the gel to the side: As we can see in the gel electrophoresis image, the expected results has been obtained. For example, Lane 8 ladder contains only the Lux0047E gene where as Lane 6 has J23002 + Lux0047E, hence the band in Lane 8 goes slightly further relative to Lane 6 because it is a shorter band. Lanes 4 and 5 show exactly the same band because they are the same genes in the same plasmid backbone. Furthermore, Lane 9 shows no band because it is the Master Mix and our negative control. Therefore, it can be concluded that the primers are functional.
Apart from the Wet Lab, I've also helped Jeremey with the summary of our Lethbridge Workshop.
Jeremy
Today I completed the ligation and transformation of the I0500+E0034 plasmid switch. I also ran the PCR products and the results came back negative. So I set up another PCR and Gel.
Patrick
Today I helped plasmid prep the degP promoter and cpxR promoter on a GFP and RFP reporter device each. I also continued looking to literature regarding high and low-copy plasmids for the modelling project.
Himika
Today I plated the construction that I did last night. I also overnight cultured the construction from last night by inoculating from the cells to be plated. Tomorrow I will plasmid prep the cultures. I also looked into the literature regarding the plasmid which will be presented to Paul on Thursday.
Chris
Today, I did a colony PCR of the CpxP promoter that was transformed into both ampicillin-kanamycin and ampicillin-chloramphenicol plasmids. The results of the colony PCR were then run on a 1.2% gel and the bands were very irregular as shown on the right. The gel was run for approximately 1 hour and the bands did not move after 35 minutes. Another colony PCR was set up and let to run again overnight. As well, overnight culures were made of all 11 samples so they could be plasmid prepped appropriately if the Colony PCR showed up possible correct bands. As well, I helped Emily test the plate reader with several cultures that had arabinose inducible promoters induced overnight of GFP and RFP.
No notebook page exists for this date. Sorry! "
"