Team:Stockholm/16 September 2010
From 2010.igem.org
Contents |
Andreas
Assembly of new parts
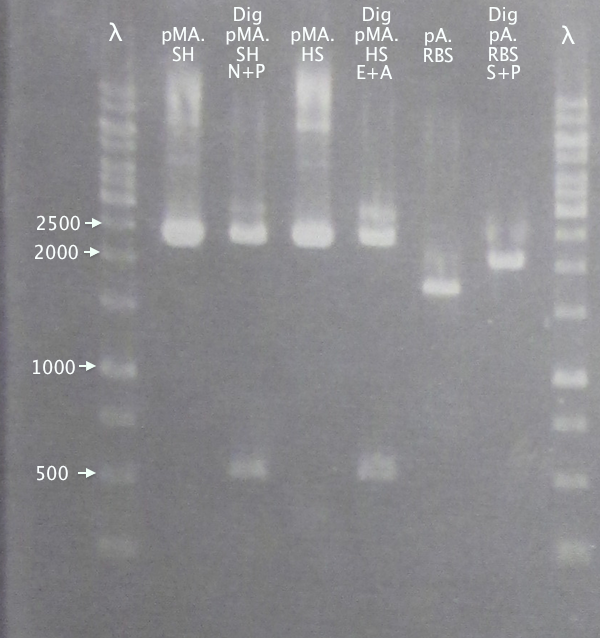
Gel verification of part digestions
Ran gels of digestion samples in parallel with undigested samples to verify successful digestions and insert sizes.
Gel 1
1 % agarose, 110 V
Gel 2
1 % agarose, 110 V
Results
Successful digestion with corresponding bands for all digested samples. N-TAT and N-Tra10 not verified, since gel was run too far, but plasmid linearization should indicate successful digestion.
Most samples show somewhat incomplete digestion. For digestions with FastDigest enzymes, this may be an indication of old/inactive enzymes. Especially PstI should be analyzed for activity.
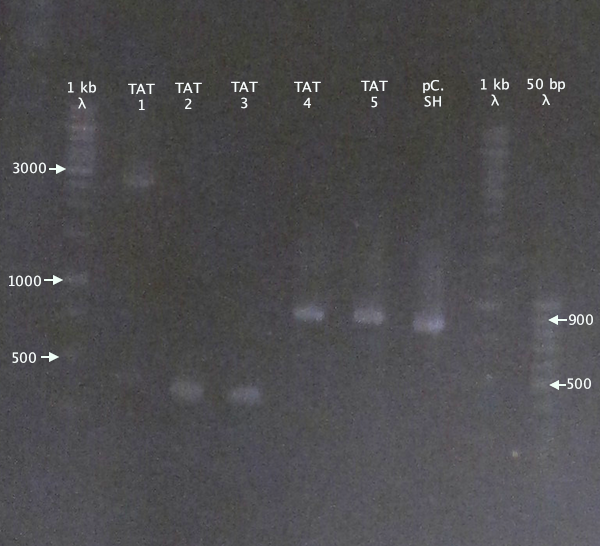
Colony PCR
Picked new colonies for colony PCR from 14/9 plates:
- pSB1K3.N-TAT⋅SOD⋅His: TAT⋅SH 1-5
- pSB1K3.N-Tra10⋅SOD⋅His: Tra10⋅SH 1-5
- pEX.SOD 1-4
Standard colony PCR settings.
- Elongation: 1:30
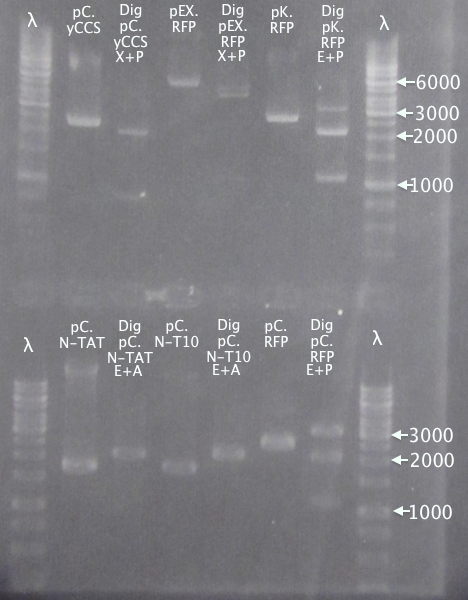
Gel verification
Gel 1
1 % agarose, 110 V
Expected bands
- pSB1K3.N-TAT⋅SOD⋅His (TAT): 848 bp
- pSB1C3.SOD⋅His (pC.SH): 815 bp
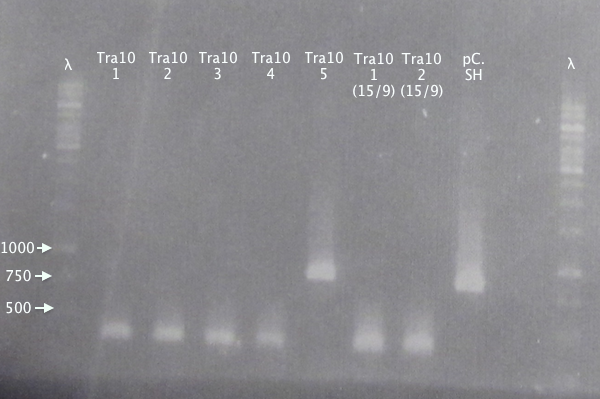
Gel 2
1 % agarose, 110 V
Expected bands
- pSB1K3.N-Tra10⋅SOD⋅His (Tra10): 878 bp
- pSB1C3.SOD⋅His (pC.SH): 815 bp
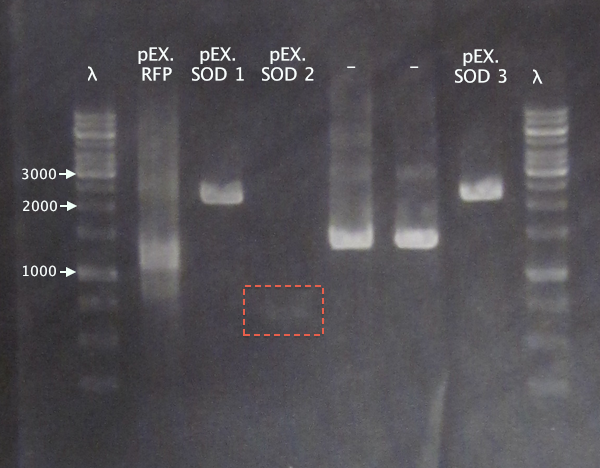
Gel 3
1 % agarose, 110 V
Expected bands
- pEX.SOD: 678 bp
- pEX.RFP: 862 bp, 1010 bp, 1124 bp, 1272 bp
Results
pSB1K3.N-TAT⋅SOD⋅His clones 4 and 5 seem slightly larger than the control, pSB1C3.SOD⋅His, indicating correct assembly.
Of the pSB1K3.N-Tra10⋅SOD⋅His samples, clone 5 seems correct, compared to the control (same as above).
For the pEX.SOD samples the results are very strange. Two of the clones resulted in way too large bands (≈2500 bp); unclear what these vectors carry. Clone 2 resulted in a very weak band with the correct size. This clone was chosen for ON growth.
ON cultures
Set ON cultures (5 ml LB + 50 Km or 100 Amp; 37 °C, 220 rpm) of the following:
- pSB1K3.N-TAT⋅SOD⋅His 4
- pSB1K3.N-TAT⋅SOD⋅His 5
- pSB1K3.N-Tra10⋅SOD⋅His 5
- pEX.SOD 2
N-CPP sequencing
Sequencing results from 13/9 returned.
- pSB1C3.nCCP 2 (fasta)
- pSB1C3.nCCP 3 (fasta)
- pSB1C3.nCCP 5 (fasta)
- pSB1C3.nCCP 8 (fasta)
- pSB1C3.nCCP 9 (fasta)
- pSB1C3.nCCP 10 (fasta)
- pSB1C3.nCCP 11 (fasta)
- pSB1C3.nCCP 12 (fasta)
Ran multiple nucleotide Blast (Blastn) alignments to identify the three N-CPPs from the sequence:
- pSB1C3.N-TAT: clones 9 & 12 (Blastn)
- pSB1C3.N-Tra10: clone 5 (Blastn)
- pSB1C3.N-LMWP: clones 2, 3 & 11 (Blastn)
ON cultures
Set ON cultures for plasmid prep (5 ml LB + 25 Cm; 37 °C, 220 rpm) and glycerol stocks (3 ml LB + 25 Cm; 30 °C).
- Clone 5: pSB1C3.N-Tra10
- Clone 11: pSB1C3.N-LMWP
- Clone 12: pSB1C3.N-TAT
Mimmi
SOD / yCCS
protein gel
- Thaw the samples and re-heat them in 95°C, 5min
- Load them on a PhastGel
- 20% 8 wells x2
| SOD | yCCS | |
|---|---|---|
| length | 154aa | 249aa |
| Ip | 6.0695 | 6.6594 |
| charge | -2.0 | +0.5 |
| size | 15.9kDa | 27.3kDa |
SOD.his 0h 1h 2h 3h his.SOD 0h 1h 2h 3h yCCS 0h 1h 2h 3h
| well | sample | well | sample | |||
|---|---|---|---|---|---|---|
| 1 | ladder | 1 | ladder | |||
| 2 | SOD.his 0h | 2 | his.SOD 2h | |||
| 3 | SOD.his 1h | 3 | his.SOD 3h | |||
| 4 | SOD.his 2h | 4 | yCCS 0h | |||
| 5 | SOD.his 3h | 5 | yCCS 1h | |||
| 6 | his.SOD 0h | 6 | yCCS 2h | |||
| 7 | his.SOD 1h | 7 | yCCS 3h | |||
| 8 | ladder | 8 | ladder |
glycerol stock / over expression
yCCS
- Pick 2 new colonies, grow ON
SOD.his/his.SOD
- Pick some cells from the glycerol-stock, grow ON
| glycerol stocks |
|---|
| yCCS 1 3ml LBAMP + 2µl culture (from PCR) |
| yCCS 2 3ml LBAMP + 2µl culture (from PCR) |
| ON for expression |
| SOD.his 1 5ml LBAMP + 5µl culture (from PCR) |
| his.SOD 1 5ml LBAMP + 5µl culture (from PCR) |
| yCCS 1 5ml LBAMP + 5µl culture (from PCR) |
| yCCS 2 5ml LBAMP + 5µl culture (from PCR) |
PCR
| mix | (µl) | x4 | Primers | conditions | ||||
|---|---|---|---|---|---|---|---|---|
| mastermix | 24.5 | pEX | time | °C | ||||
| DNA | 0.5 | 10m | 95 | |||||
| tot | 25µl | 30s | 95 | ) | ||||
| 30s | 55 | > 30 cycles | ||||||
| 1m | 72 | ) | ||||||
| 5m | 72 | |||||||
| oo | 25 | |||||||
MITF-M
colony PCR
| mix | (µl) | x8 | conditions | |||
|---|---|---|---|---|---|---|
| mastermix | 24.5 | time | °C | |||
| DNA | 0.5 | 10m | 95 | |||
| tot | 25µl | 30s | 95 | ) | ||
| 30s | 55 | > 30 cycles | ||||
| 2m | 72 | ) | ||||
| 10m | 72 | |||||
| oo | 25 | |||||
Gel
| well | sample |
|---|---|
| 1 | ladder |
| 2 | MITF-M 1 |
| 3 | MITF-M 2 |
| 4 | MITF-M 3 |
| 5 | MITF-M 4 |
| 6 | MITF-M 5 |
| 7 | nTAT |
| 8 | cTAT |
| 9 | nTra10 |
Johan
PCR
- 15sep pMA AS bFGF NS
- 15sep pMA EN bFGF EA
0,5 µl Pol
0,5 µl dNTP
5 µl 5x buffer
1,5 µl for primer
1,5 µl rev primer
16 µl H2O
3 colonies/plate
 |

|
 |

|
 |

|

|

|
 "
"