Team:Cambridge/Notebook/FurtherWork
From 2010.igem.org

Viewing project diary
View protocols and data
|
By this stage term was about to start and members of the team had very little time. Nevertheless, due to the synthesis dramas described, work had to continue for some time.
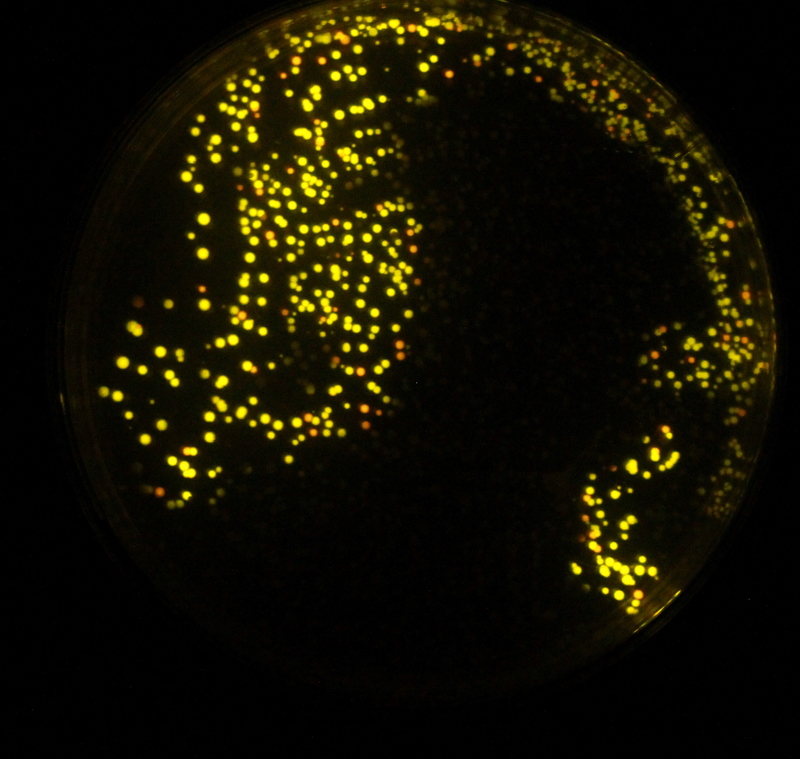
Monday 4th OctoberToday we received extremely bad news. We had ordered 3 DNA constructs for synthesis by Mr. Gene on 19th August who had said that 98% of sequences of this length would have a turn-around time of 14 business days. We had become increasingly alarmed as time had gone on without the order being completed. We received our first contact from them at this time, informing us that the company was having problem with one of our constructs - luxAB. They said they had split the sequence into three parts and had to start from scratch, and that it might be 3 weeks until we got the sequence. This would take us until just before the Jamboree. We moped for a bit and then came up with a plan, we asked them to send the completed sequences as soon as possible. We decided we would attempt to fuse them to the Xenorhabdus luminescens luxAB submitted by last year's Edinburgh team. But there was good news as shown by the image on the right. We had believed that a number of our colour changes had failed due to sequencing results. But when we assayed our plates we found that some colonies had the desired phenotype. The plate on the right shows two distinct colour types. Tuesday 5th OctoberWe spent much of today moving out of the lab, we also analysed the results of the plate reader experiment. Luciferin can be visualised by its fluorescence under UV light as shown on the right. Wednesday 6th OctoberOur term proper began today so we were mostly busy but Paul came into the lab. Thursday 7th OctoberBen miniprepped G28 and some of the colour-changed luciferases for sequencing and placed the order. Emily and Peter transformed two hns-mutant strains (black and red) with G28 and of Top10 with CD and EG from Mr Gene. In case that didn't work, we should have plenty of DNA left in the tube they sent us. I'll check in on those tomorrow. The Top10 cells are plated on kanamycin plates. If the transformation works, we can roll out the Gibson assembly. Assemblies to do are: CD into Psb1C3 EG into Psb1C3 CD+EG into Psb1C3 pBad+CD+EG into Psb1C3
Saturday 9th OctoberPeter went in to check on the transformation plates today, the CD and EG transformants grew up massively, i.e. hundreds of cultures. There are still a couple of singles, so we should be fine (unless our Soc is contaminated again). The hns mutants (termed R28 and K28, if only to abuse Will's naming system) don't show any colonies yet, but that was to be expected. 20°C and all that. Sunday 10th OctoberPeter set on liquid colonies of Top10 G28, R28, K28 (the hns mutants) pBad+PP+YFP and pBad+YFP. All of these grew up, but they were rather thin (because shaking incubator < rotation incubator and all the cells sedimented). He left them to grow a bit more and will go and take them out of the incubator tonight. There were lots and lots of colonies on the hns-mutant plates, so many to make us doubt our SOC, so Peter plated some out and left the bottle on the bench, but nothing grew over night, so we're probably fine. The K28 plate was grown over quite densely, so it was put in the fridge yesterday. Peter checked both plates for glowth yesterday and couldn't see anything, but today the R28 plate (a hns knockout) glowed quite well, so yay! everything is in the fridge now. Tuesday 12th OctoberPeter went into the Plants lab and looked at the results of the plate reader. All the strains seem to be growing fine, with quite consistent ODs, Luminescence has fallen off in all of them by now and seems to have leveled out. Three wells, however gave a massive spike at the start, much brighter than all the others. 'Which one?', I hear you ask, 'is it K, the master of quiescence, R, the loveable knockout, or G, the old champion?' For now, imagine the plate reader hanging off a cliff... Can our cells overcome the repression and shine brighter than they ever shone before? Will science happen? Are hns-mutants shite? Tune in to watch the exciting conclusion of Plate Reader: The Reckoning! Wednesday 13th OctoberPreviously on Plate Reader: The Reckoning (title: Based on a true story) Paul: "Lets set up the machine to read K28 and R28 brightness at different arabinose concentrations. It's a long shot, but it might just work" Bill: "You're crazy! we need to run calculations, replicates, controls! We'll need at least 5 days to prepare everything!" Paul: "You've got 5 minutes" Bill: "I'll do it in 2" (Montage of 72 wells being loaded with three replicates at each concentration and one row of controls, G28 at different arabinose concs and blanks) Paul: "Go my mutants! To a brighter future, free from repression!" (cut to black, sounds of OD readings) And now: (dedicated to the lives lost in transformation) At the start, K28 seemed a bit disoriented and didn't shine very bright. R28 made and honest effort, but neither could step out of the shadow of G28, which surged to a peak of 3.7 million units of pure awesomeness after a few hours. But soon after that, G28's btightness came crashing down. K28 still hadn't quite got to grips with the situation - while growing happily, it didn't seem into the whole glowing business. But what is that? Even though only half as bright as G28 at the start, R28 takes a stand against the fall and maintains its luminescence at a respectable level of 1.7 million Somethings. G28 hadlong fallen into darkness, but R28 is still going strong to this hour. K28 went to get a coffee...
Right, like any season finale this gave more questions than answers. My take on all of that would be that H-NS does indeed repress, but is still affected by physiological changes connected with cell density, despite the absence of the Vibrio quorum sensing system. Therefore, expression is reduced after a while and falls off rapidly. In R28, the lux operon is not repressed and keeps glowing away as the cells are growing and slowly changing into stationary phase. The initially lower brightness might be due to greater availability of secondary substrates, like ATP, as Top10 is generally more viable. Another explanation could be that G28 burns out all its resources at the start, while R28 lives at a much slower pace (although the OD values do not show this), but uses a greater proportion of its resources to make light. K28 just sucks. We left the plate reader running. It will be interesting to see how long R28 is able to maintain its light output. Either way, there definitely is a significant influence of H-NS on LuxCDABEG without the original promoter regions, and we can definitely present that as evidence for our codon-usage-change approach. Peter bumped into Jim Ajioka today and he asked me to let him know when we are going to do the LRE/spinning down experiment. Are we still going to do that? It would help us a fair bit in showing the efficacy of LRE and how much of the effect depends on liberation of luciferase and how much is actual recycling. Thursday 14st OctoberBasically, the sequencing didn't work. There was something wrong with the sample concentrations or something, so even though they say they've repeated the readings they can't get any meaningful results. So we don't yet know if we have successful colour changes yet I'm afraid. BUT, all we need to do is send some more samples off. I'm afraid I'm busy today so can't. Any volunteers? Duncan has also asked to remind us all about trying to organise that lab meeting with the supervisors next week. Thursday 21st OctoberWill and Peter had a long day in an attempt to do a number of Gibson Assembly operations. The most crucial was to place Edinburgh's luxAB under pBAD and then add our codon optimised CD and EG to it. | Team:Cambridge/LabBook/Week13 |
 "
"