Team:ETHZ Basel/Modeling/Light Switch
From 2010.igem.org
Modeling of the relocation of the proteins by red and far-red light
We relocate one of the Che proteins by fusing it either to PhyB or to PIF3. To model the relocation, we decided to try different approaches. One is based on the model recently published by Sorokina et al [1]. As a first step we reimplemented the model as published in the data.
General remarks on the model of Sorokina et al
In [1] Sorokina et al developed (in vivo) and modeled (in silico) the reversible activation of different phytochromes by red and their deactivation by far red light in yeast. Their main model is based on the proteins PhyA and FHL, which have similar properties as PhyB and PIF3 used in our system. In their system PhyA is fused to the Gal4 bindig domain (GBD) and FHL to the Gal4 transcriptional activator domain. Upon activation with red light, both chimeric proteins bind to each other and the complex can activate the transcription of a lacZ reporter which is under the control of a GAL4-responsive artificial promoter. Upon deactivation with far-red light, the complex dissociates and transcription of the lacZ gene is significantly reduced.
In the model Sorokina et al assume that the PhyA protein sequesteres and that only free PhyA can interact with FHL. They state that this interaction is necessary to explain their measurement data, consisting of the strength of fluorescence induced by lacZ ofer time for different experimental settings. Please remark that this signal changes with time constant in the range of hours.
Implementation
One of the models of the system by Sorokina et al [1] is available in the SBML format ([http://www.biomedcentral.com/content/supplementary/1754-1611-3-15-S3.XML]). We exported the model to Matlab using the SBToolbox2 ([http://www.sbtoolbox2.org]). As a first step we tried to reproduce the data presented in the article [1]. However, this task was not trivial since, although the model is available in the SBML format, necessary parameters are missing. The model as downloaded can only reproduce qualitatively the data for the system kept in the dark. The parameters necessary to define the light input signals were missing.
We tried to reverse engineer these parameters from the information given in the paper. However, we had to recognize that the model available in SBML was slightly different from the model as described in the article: Different to the model in the article, it is not possible to set the strength of the red and far-red light signals directly, but one has to set their derivatives. We believe that this modeling decision might be made to circumvent the problem that some integrators fail to solve a system of differential equations correctly when facing a too high step change in one of the derivatives. To test the model, we removed this work-around and simply not changed the the light inputs by steps, but by ramps with a length of 1s, which is significant smaller than the other time constants being relevant for this part of the model. When we then tried to reproduce the data in the paper, we recognized that we had to reduce the strengths of the light signals approximately by 40 fold. Since we do not know how exactly the inputs were set in the original simulations, we could not deduce the reason for this necessary change in the parameters of the model.
Furthermore several other parameters were not available. These parameters were mainly needed to calculate the fluorescence signal strength from the lacZ concentration. In the paper [1] it is stated that these parameters were obtained for every experiments separatly by fits. To evaluate if the model can reproduce the data in the paper, we estimated one of the more important parameters (RLU in [1]) and did set the other parameters to the standard values from the SBML model, since changes of these parameters had similar effects.
Evaluation of the model
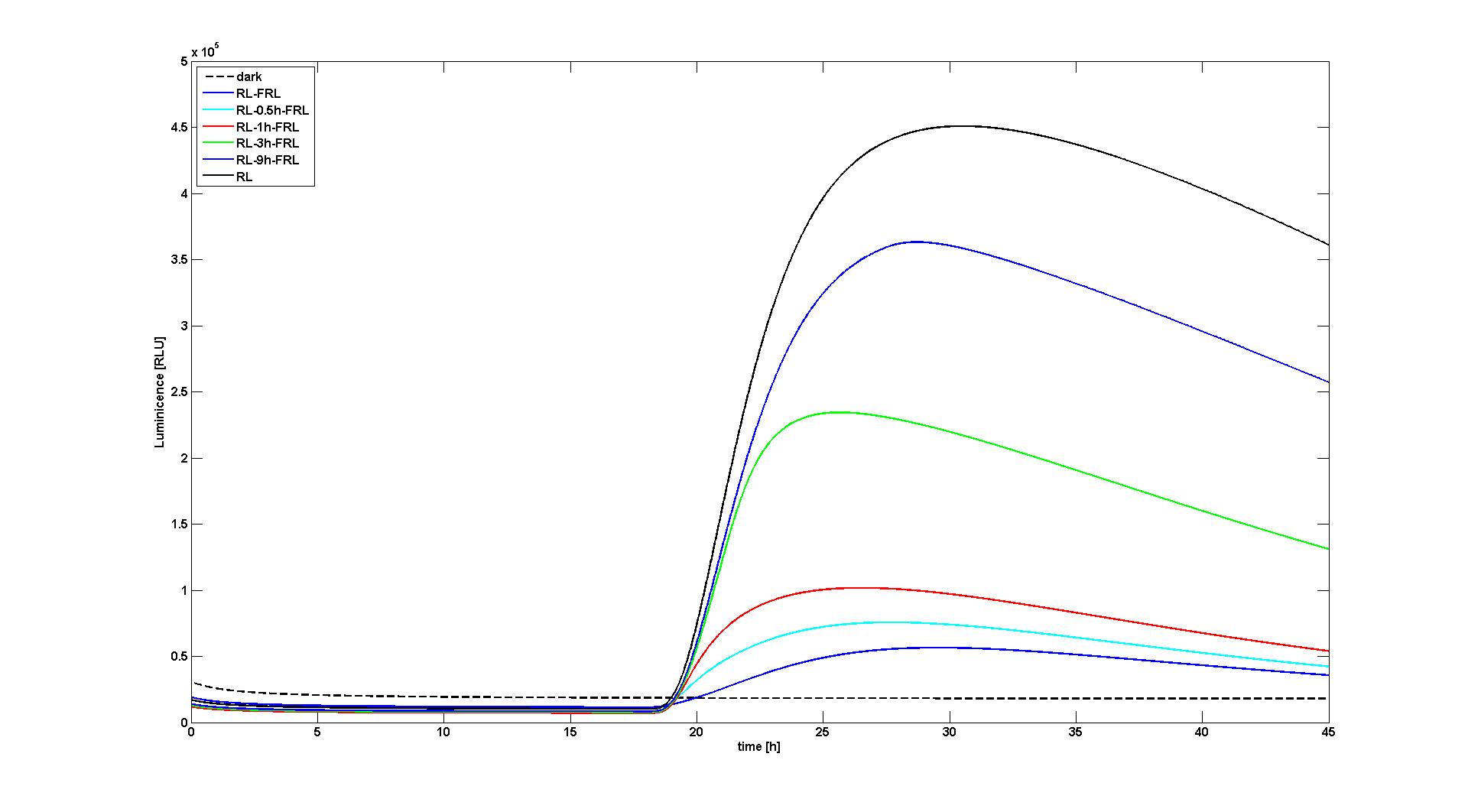
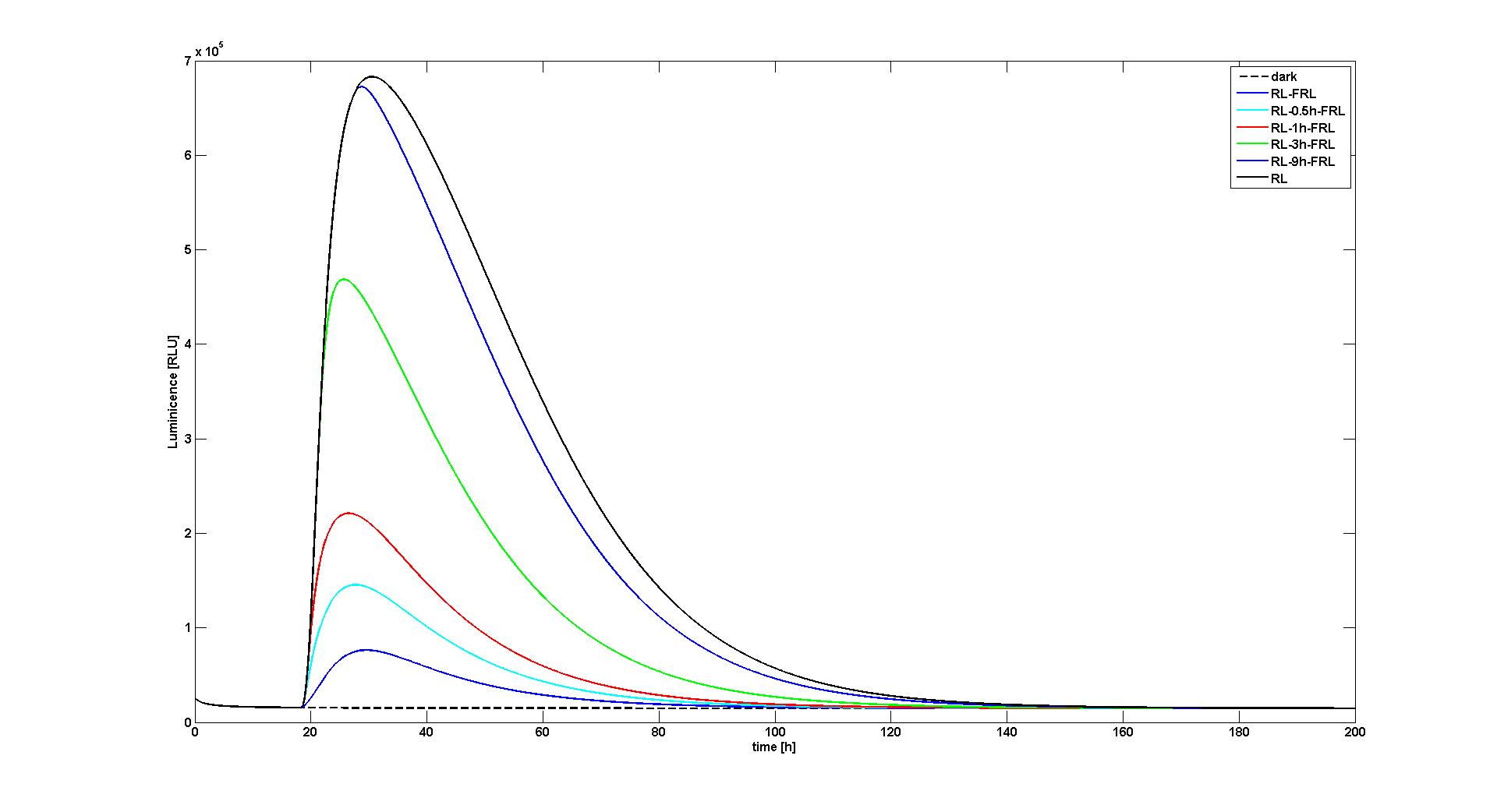
With the changes made to the model we were able to reproduce the data as presented in the paper. We reproduced two of the most significant figures (see Figure 1 and 2), which show the response of the system to different light input signals: Wither to no light input at all (dark), a single red light pulse at t=18h lasting for 10 min, or a 10 min red light pulse at t=18h followed by a 10 min far-red light pulse after 0.5h, 1h, 3h, or 9h. All experiments qualitatively and quantitaively reproduce the simulation results obtained by Sorokina et al (see Figure 6B and Figure 8A in [1]).
Concerns about the model
The data in the paper of Sorokina et al [1] could only be reproduced with changes in the strength of the light inputs. Although with these changes we can now reproduce the data, it is not clear how exactly the original simulations were produced and if these changes are valid or if the light inputs were set differently by Sorokina et al (it is not non-ambiguous how exactly the inputs in the model have to be set). However, this part of the model is substantial for us.
Furthermore Sorokina et al state that the sequestered state is necassary to reproduce the longevity of the responses of the system to light pulses. They validated it by showing that a lower lacZ protein degradation rate only changes the height, but not the shape of their output. However, it is easy to change the shape/longevity of the outputs by altering the mRNA degradation rate. The rate they used for the experiments was obtained by dark experiments only. It is questionable if this rate is identifiable by those experiments, which cannot be checked by us. More crucial, Sorokina et al state that this rate (and other rates of the LacZ system) were not part of the fitting of their models to data obtained by experiments with changing light strengths.
We are not in the position to question the validity of the model by Sorokina et al, since we have not all the data used to construct the model. It can well be that this data is convincing enough so that we could use their model. However, in a combination with the uncertainty how exactly to set the light inputs in the simulations to reproduce the data, this concerns suggest that we should not stay to closely to their modeling decisions, exspecially concerning the sequestering strength of PhyA.
Status
Please find the current version on the SVN-Server.
- (7/25): (SVN rev. 3) start development by George
- (7/27): (SVN rev. 4) original model partly implemented, not yet working (Goal 1.1)
- (7/28): (SVN rev. 5) original model partly implemented, so far working: Goal 1.1 reached
- (7/31): (SVN rev. 6) first attempts to set light-conditions according to experimental setups (Goal 2)
- (8/03): (SVN rev. 7) simplifications, control of all equations and parameters (Goal 2)
- (8/07): (SVN rev. 8) first try to connect light-switch to chemotaxis model (Goal 3)
- (8/10): (SVN rev. 10) goal 2 reached (Goal 2)
- (8/15): Moritz takes over modeling work due to to the exams of George.
- (8/19): Testing the models so far. Models do not reproduce the data. Resetting all goals to not-reached.
- (8/20): Failures found and corrected. Goal 1 and 1.1 reached. Possibility that original model might not be the best: Light input in original model modelled suspicious. Had to reduce light strength by 40 fold (how they exactly modeled the light is not completely clear. Possible that they explained it ambiguously in the paper). Do not believe in data for the sequestered state.
- (8/20): Redefinement of goal: Get origins of the parameters used by Sorokina et al. Build own trusted model.
References
[1] [http://www.jbioleng.org/content/3/1/15 Sorokina et al: A switchable light-input, light-output system modelled and constructed in yeast. J Biol Eng. 2009 Sep 17;3:15.]
 "
"


