|
AUGUST: WEEK 2
August, 9th
Phasins plates showed very few colonies (12 for I20-new and 17 for I21-new): they were all picked and let grow in LB+Amp 100ug/ml. In the evening we made glycerol stocks and re-filled the tubes for the screening of the following day:
| I20-new: | I21-1 | I21-2 | I21-3 | I21-4 | I21-5 | I21-6 | I21-7 | I21-8 | I21-9 | I21-10 | I21-11 | I21-12
|
| I21-new: | I20-1 | I20-2 | I20-3 | I20-4 | I20-5 | I20-6 | I20-7 | I20-8 | I20-9 | I20-10 | I20-11 | I20-12 | I20-13 | I20-14 | I20-15 | I20-16 | I20-17
|
In order not to loose our time we decided to perform again the PCR-amplification/modification of <partinfo>BBa_K208001</partinfo> in case of a negative screening. Than we gel-ran and gel-extracted right amplicons.
PCR was performed with:
- 10_F and S_R primers
- S_F and S_R primers
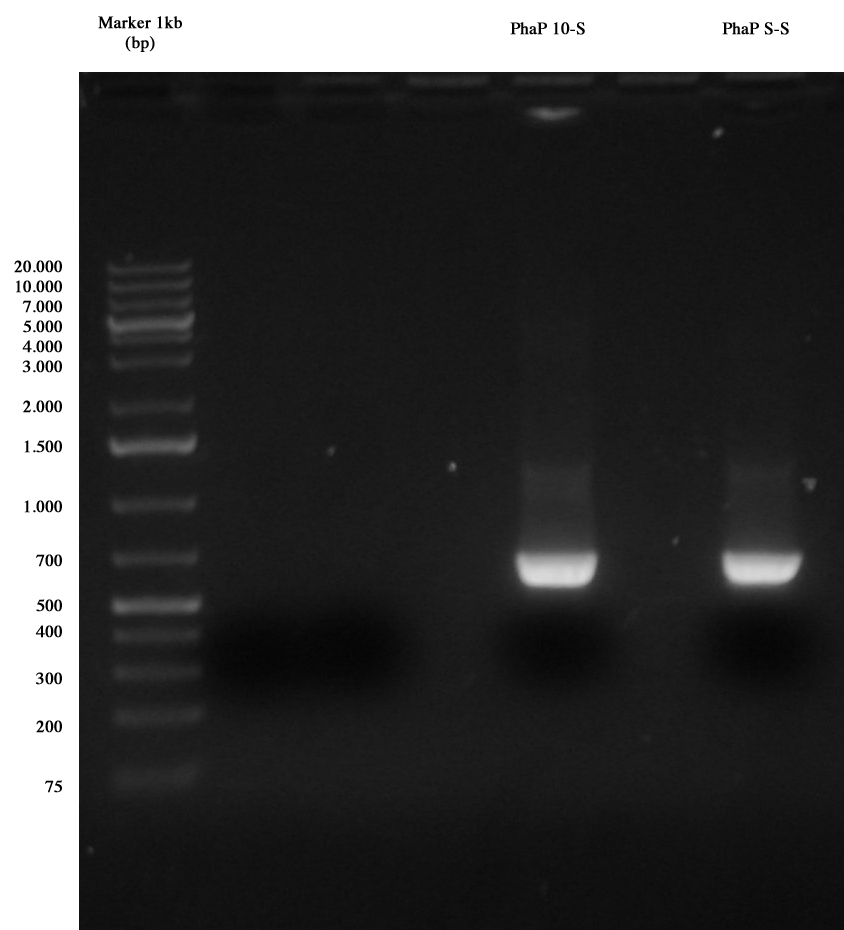 PCR results: PhaP 10-S and PhaP S-S The image shows results of our PCR: both amplicons are positive, so we purified cut DNA, obtaining the following quantifications:
- PhaP 10-S: 16,8 ng/ul
- PhaP S-S: 17,1 ng/ul
Tomorrow we will proceed to digest this part, in order to have an alternative if all clones of I20 and I21 are negative.
In the afternoon, we inoculated all parts we will need tomorrow.
| Part | Medium | Task
|
| <partinfo>BBa_B0034</partinfo> | 5ml LB+Amp | Ligation
|
| I15-1 | 5ml LB+Amp | Ligation
|
| Linker | 5ml LB+Amp | Ligation
|
| pSB4C5 | 5ml LB+Cm 12,5 | Ligation
|
| I22-1 | 5ml LB+ Amp (picked from colony) | Ligation/Screening/Glycerol stock
|
| I22-2 | 5ml LB+ Amp (picked from colony) | Ligation/Screening/Glycerol stock
|
| I22-3 | 5ml LB+ Amp (picked from colony) | Ligation/Screening/Glycerol stock
|
| I23-1 | 5ml LB+ Amp (picked from colony) | Screening/Glycerol stock
|
| I23-2 | 5ml LB+ Amp (picked from colony) | Screening/Glycerol stock
|
| I23-3 | 5ml LB+ Amp (picked from colony) | Screening/Glycerol stock
|
| <partinfo>BBa_r0062</partinfo> | 5ml LB+ Amp | Ligation
|
| I7_4C5-2 | 1ml LB+ Cm12,5 | Tecan Test
|
| I8_4C5-2 | 1ml LB+ Cm12,5 | Tecan Test
|
| I10_4C5-1 | 1ml LB+ Cm12,5 | Tecan Test
|
| I12_4C5-1 | 1ml LB+ Cm12,5 | Tecan Test
|
| A2(=<partinfo>BBa_J23100</partinfo>+GFP) | 1ml LB+ Amp | Tecan Test
|
| <partinfo>BBa_B0032</partinfo> | 1ml LB+ Amp | Tecan Test
|
| I8-5 D | 1ml LB+ Amp (picked from colony) | Tecan Test/Glycerol stock
|
| I8-5 E | 1ml LB+ Amp (picked from colony) | Tecan Test/Glycerol stock
|
| I8-5 F | 1ml LB+ Amp (picked from colony) | Tecan Test/Glycerol stock
|
Check of LB+Cm 6 ug/ml agar plates. We let grow at 30°C:
- MG1655 in LB
- MC1061 in LB
- MG123/008 in LB+Amp 50 ug/ml
- MC123/008 in LB+Amp 50 ug/ml
- F2620-4C5 (positive control)
Than we streaked cultures on a seven-sector divided plate, and we let grow it ON at 30°C. Since 6 ug/ml is a very low concentration, we wanted to check if actually nothing (except F2620-4C5) grow on these plates.
Transformation at 30°C of 100 ul of MG13/008 and MC123/008 competent cells to check their efficiency. We used 1 ul (~4 ng) of RING, F2620-4C5, Nothing (water) and plated on proper LB agar plates:
- RING: Cm 34 ug/ml
- F2620-4C5 (positive control): Cm 12,5 ug/ml
- Nothing (negative control): Cm 12,5 ug/ml
Both RING and F2620-4C5 should survive, but we had a problem so that MC008 transformed with RING couldn't be plated. We will check it another time. We let grow plates ON, 30°C.
August, 10th
Glycerol stock was prepared for:
- linker
- I22-1
- I22-2
- I22-3
- I23-1
- I23-2
- I23-3
- I8-5 D
- I8-5 E
- I8-5 F
and are stored at -80°C.
Cultures were diluted (5ul in 2ml LB+antibiotic) for TECAN test:
| I8-5 D | I8-5 E | I8-5 F
|
| I74C5 | I84C5 | I104C5
|
| I124C5 | Rbs32 | A2
|
MiniPrep was performed for following cultures (using our new NucleoSpin kit, that we prepared yesterday! :) ):
| Culture | Quantification
|
| I20-1 | 16,5 ng/ul
|
| I20-2 | 8,9 ng/ul
|
| I20-3 | 19,3 ng/ul
|
| I20-4 | 12,5 ng/ul
|
| I20-5 | 22,4 ng/ul
|
| I20-6 | 12,3 ng/ul
|
| I20-7 | 4,1 ng/ul
|
| I20-8 | 15,7 ng/ul
|
| I20-9 | 14,4 ng/ul
|
| I20-10 | 17,5 ng/ul
|
| I20-11 | 24,5 ng/ul
|
| I20-12 | 21,2 ng/ul
|
| I21-1 | 22,2 ng/ul
|
| I21-2 | 24,6 ng/ul
|
| I21-3 | 14,2 ng/ul
|
| I21-4 | 12,4 ng/ul
|
| I21-5 | 19,4 ng/ul
|
| I21-6 | 17,0 ng/ul
|
| I21-7 | 14,5 ng/ul
|
| I21-8 | 23,1 ng/ul
|
| I21-9 | 18,9 ng/ul
|
| I21-10 | 16,4 ng/ul
|
| I21-11 | 13,3 ng/ul
|
| I21-12 | 15,5 ng/ul
|
| I21-13 | 33,5 ng/ul
|
| I21-14 | 22,2 ng/ul
|
| I21-15 | 21,1 ng/ul
|
| I21-16 | 23,8 ng/ul
|
| I21-17 | 20,6 ng/ul
|
| I22-1 | 117,0 ng/ul
|
| I22-2 | 100,4 ng/ul
|
| I22-3 | 281,3 ng/ul
|
| I23-1 | 55,2 ng/ul
|
| I23-2 | 47,1 ng/ul
|
| I23-3 | 84,8 ng/ul
|
| I15-1 | 80,7 ng/ul
|
| Ent4C5 | 27,6 ng/ul
|
| rbs34 | 50,4 ng/ul
|
| linker (<partinfo>BBa_K105012</partinfo>) | 39,3 ng/ul
|
| plux | 45,1 ng/ul
|
Digestion of:
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 | Enzyme 2 | Buffer H
|
| I20-1 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I20-2 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I20-3 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I20-4 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I20-5 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I20-6 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I20-7 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I20-8 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I20-9 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I20-10 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I20-11 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I20-12 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I21-1 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I21-2 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I21-3 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I21-4 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I21-5 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I21-6 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I21-7 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I21-8 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I21-9 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I21-10 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I21-11 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I21-12 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I21-13 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I21-14 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I21-15 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I21-16 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I21-17 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I22-1 | Insert/Screening | 25 | 17 | 3,5 | 1 XbaI | 1 PstI | 2,5
|
| I22-2 | Insert/Screening | 25 | 20,5 | 0 | 1 XbaI | 1 PstI | 2,5
|
| I22-3 | Insert/Screening | 25 | 7,1 | 13,4 | 1 XbaI | 1 PstI | 2,5
|
| I23-1 | Insert/Screening | 25 | 10 | 10,5 | 1 XbaI | 1 PstI | 2,5
|
| I23-2 | Insert/Screening | 25 | 10 | 10,5 | 1 XbaI | 1 PstI | 2,5
|
| I23-3 | Insert/Screening | 25 | 10 | 10,5 | 1 XbaI | 1 PstI | 2,5
|
| I15-1 | insert | 25 | 20,5 | 0 | 1EcoRI | 1 PstI | 2,5
|
| Entero4C5 | Vector | 25 | 20,5 | 0 | 1 EcoRI | 1 PstI | 2,5
|
| <partinfo>BBa_B0034</partinfo> | Insert | 25 | 20,5 | 0 | 1 SpeI | 1 PstI | 2,5
|
| <partinfo>BBa_K105012</partinfo> | Insert | 25 | 20,5 | 0 | 1 SpeI | 1 PstI | 2,5
|
| <partinfo>BBa_R0062</partinfo> | Insert | 25 | 20,5 | 0 | 1 SpeI | 1 PstI | 2,5
|
| PhaP 10-S | Insert | 30+10° | 26 | 0 +7 | 1+1 XbaI | 1+1 SpeI | 3+1
|
| PhaP S-S | Insert | 30 | 26 | 0 +7 | 1+1 XbaI | 1+1 SpeI | 3+1
|
| pSB1A2 | Insert | 25 | 14 | 6,5 | 1XbaI | 1 SpeI | 2,5
|
°(after '+' added after 2 hours and incubated for further 2 hours)
August, 11th
August, 12th
August, 13th
August, 14th
August, 15th
|
|