Team:ETHZ Basel/Biology
From 2010.igem.org
Overview
The goal of our iGEM project E.lemming is to take over the control of the tumbling frequency of E. coli. This is achieved by reversibly localizing certain elements of the chemotactic pathway (che-proteins) and thus affecting their activity.
For localization we use the PhyB-PIF3 system found in plants. PhyB or PIF3 is fused to a che-protein while the other one is fused to a localized protein (localizer) within the cell. Upon a light stimulus of a certain wavelength PhyB is activated, binds PIF3 and thereby localizes the che-protein. A light stimulus of a different wavelength reverses this process.
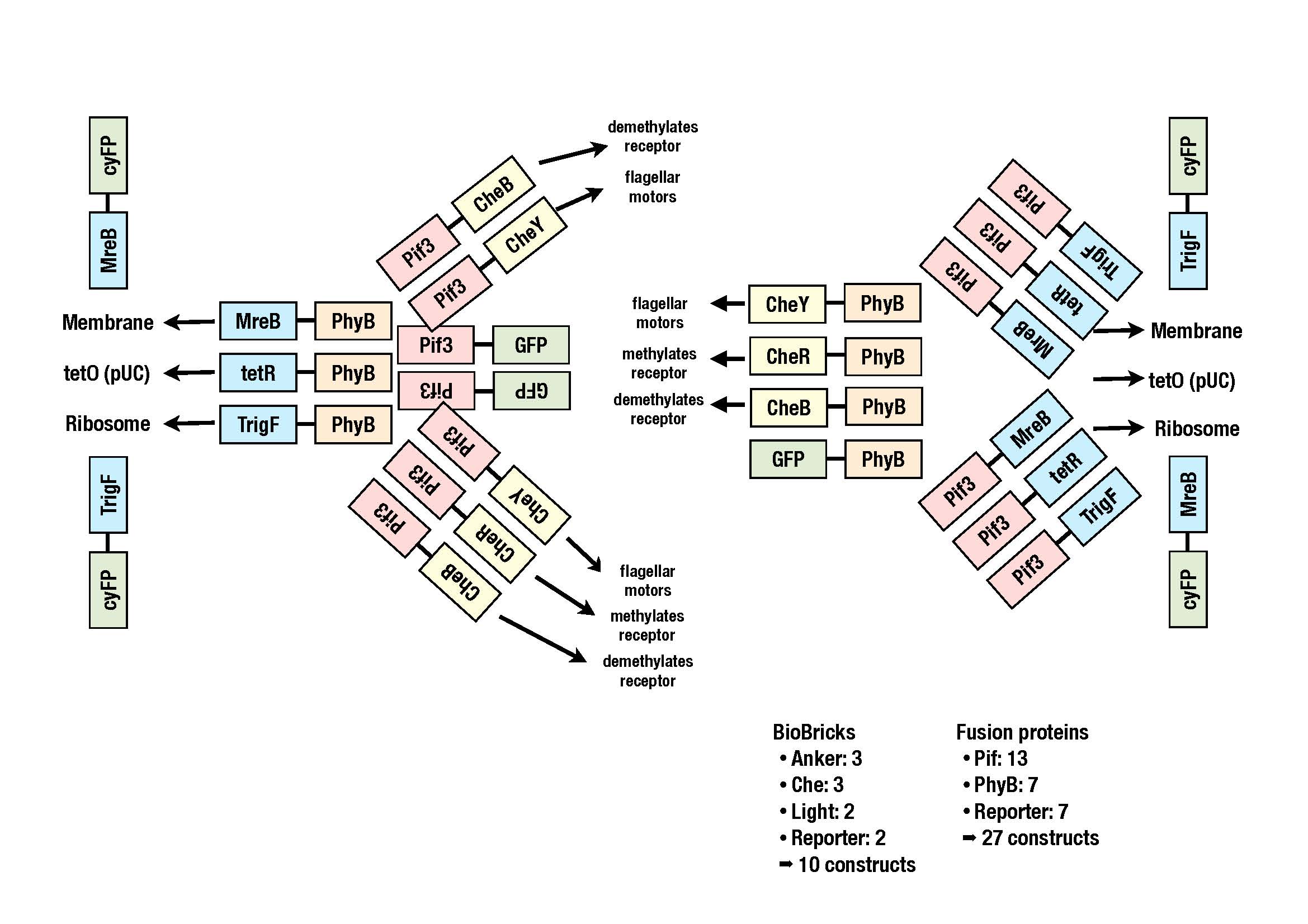
In order to increase the probability that the system will work we will try out a lot of combinations of several proteins:
- che-proteins: cheY, cheB and cheR
- localizer: trigger factor (binds the ribosome), MreB (actin analogue), tetR (binds tetO)
The goal of the wet lab team is to implement this localization system into E. coli.
Cloning Strategy
As we plan to generate several fusion proteins with different linkers, we decided to use the cloning strategy BBF RFC 28: A method for combinatorial multi-part assembly based on the Type IIs restriction enzyme AarI (http://dspace.mit.edu/handle/1721.1/46721). The advantage of this strategy is that we can clone up to 3 different inserts into one vector simultaneously in a 96 well format.
Parts
Currently we are working on putting all the BioBricks into a storage vector. The image shows all the constructs we plan to clone.
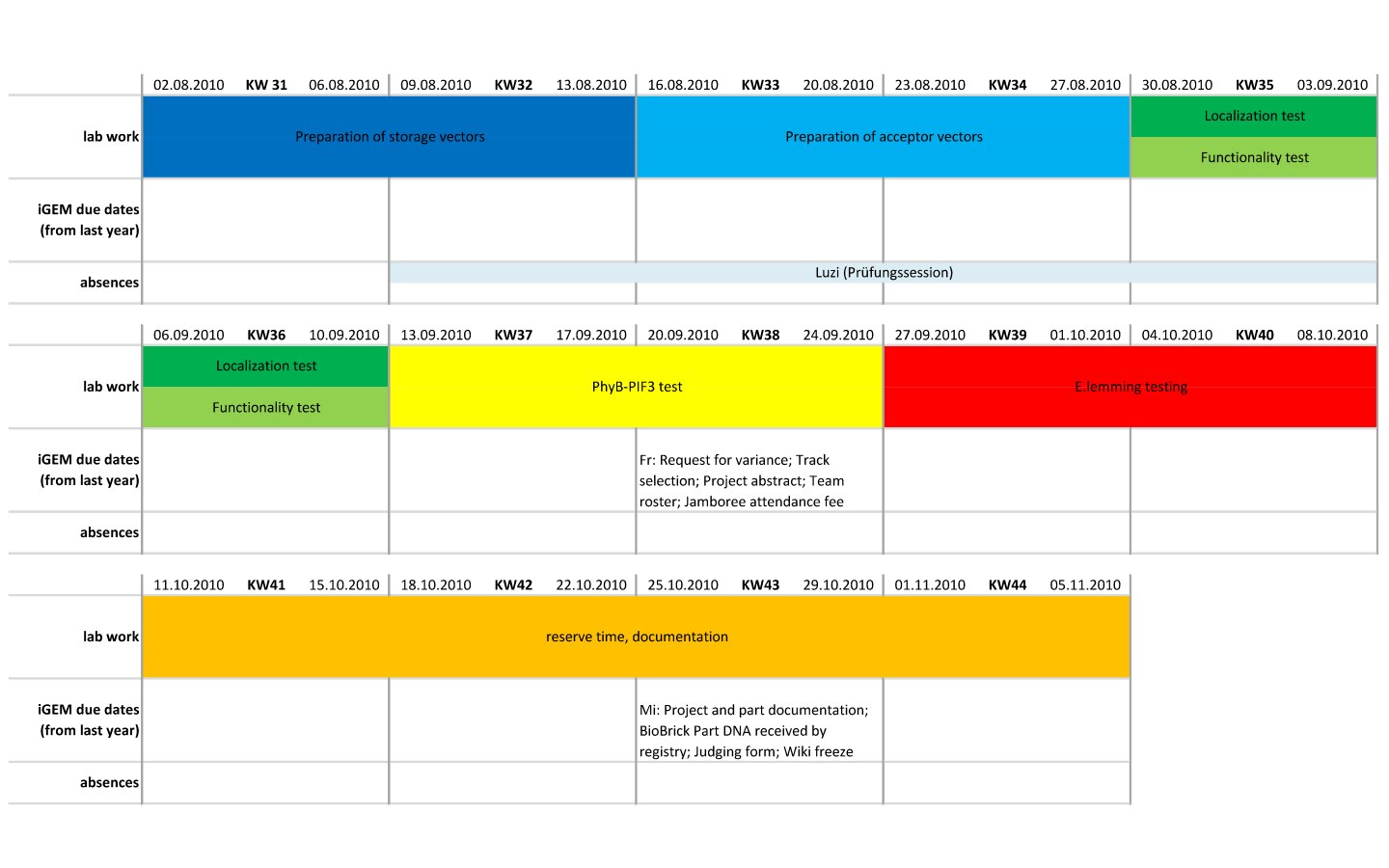
Working Process
generation of parts
generation of storage vectors
We use the vector pSEVA of Victor de Lorenzo's lab. It has a kanamycin resistance and a BBR1 origin.
The working process for the generation of the storage vectors is as follows:
Ordering of primers (if template is available) -> PCR -> clean-up of PCR product -> ligation into storage vector -> transformation of competent cells -> plating of cells -> selection of clones (blue-white-screening) -> sequencing
For proteins for which no template is available we let the let the genes synthesize directly.
| Protein | terminus on fusion | status |
|---|---|---|
| CheY | N | sequencing |
| CheY | C | sequencing |
| CheR | N | sequencing |
| CheR | C | sequencing |
| CheB | N | sequencing |
| CheB | C | sequencing |
| TrigF | N (binds N-terminal to ribosome) | PCR |
| MreB | N | ligation |
| MreB | C | ligation |
| tetR | N | ligation |
| tetR | C | ligation |
| PhyB | N | ligation |
| PhyB | C | ligation |
| PhyB | N | ligation |
| PhyB | C | ligation |
| PIF3 | N | ligation |
| PIF3 | C | ligation |
| YFP | N | |
| YFP | C | |
| GFP | N | |
| GFP | C |
generation of acceptor vectors
Once the storage vectors are created the acceptor vectors can be generated according to the cloning strategy BBF RFC 28.
testing of parts
We will have to test our constructs for the following properties:
- Fusion of che-protein and PhyB or PIF3 for chemotactic functionality
- Fusion of PIF3/PhyB and localizer for proper localization
Chemotactic Functionality
Is our fusion protein still able to maintain its function in the chemotactic pathway? To answer this question we plan to use either a swarm test.
Localization
Is our localizer directing PIF3 or PhyB sufficiently to a certain area within the cell? We will use fluorescence microscopy to test for this. To do so we will need an additional fusion protein containing a fluorescent protein. We decided to use GFP and alternatively cyFP because they shouldn’t interfere with our coupling system of PhyB and PIF3 which uses red and far red light. If the fusion between the localizer the fluorescent protein is attracted to a specific site chances are high that a fusion of localizer and PIF3/PhyB also will.
 "
"