Week of 6/14
From 2010.igem.org
June 14th
- Flasks mini prepped
-J37033 (LuxR + RBS) -C0261 (LuxI + RBS) -R0061 (Lux Promoter) -J23116 (Constitutive Promoter) -R0063 (Lux Promoter) -K145150 (Lux Promoter) -K091146 (Lux Promoter) -K274100 (Pigment) -J23108 (Constitutive Promoter)
- All of the mini preps were digested with the following:
-J37033 (XP) -C0261 (XP) -R0061 (ES) -J23116 (ES) -R0063 (ES) -K145150 (ES) -K091146 (ES) -K274100 (ES) -J23108 (ES)
- We ran the digest as usual: 1 hour in the water bath, 20 minutes heat kill. We did not phosphotase any of these as you can see all of these parts appear on the gel.
- The order was Ladder, J23108, K274100, K091146, K145150, R0063, J23116, R0061, C0261, and J37033.

- This gel came out well with the exception of K145150.
- We then cut out each available band, purified, ligated, and finally transformed.
June 15th
- Flasks mini prepped
-J37033 (LuxR + RBS) -C0261 (LuxI + RBS)
- All of the mini preps were digested with the following:
-J37033 (XP) -C0261 (XP)
- The digests were put into a 37 degree Celsius water bath for an hour. The reaction was heat killed for 20 minutes in an 80 degree Celsius water bath.
- The two parts were then run on a gel in this order: Ladder, PFTV (another student's project), C0261, and J37033.
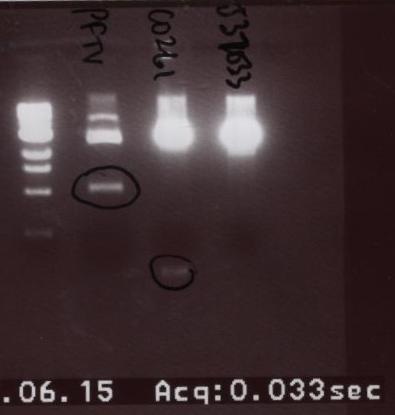
- This time the parts came out very vibrantly. We extracted C0261, did the gel purification, ligated, and transformed.

 "
"