Team:Stanford/Research/Modeling
From 2010.igem.org

| Home | Project | Applications | Modeling | Parts | Team | Notebook |
Contents |
Goals
Our intuition for what makes a good ratio sensor could only take us so far. From the very first stages of design, we wanted to back up and test our ideas with mathematical tools. Luckily, we found that solving the equations of mass action kinetics at steady-state was enough to give us clear design criteria. We present the mathematical basis for sensors that are capable of sensing a single ratio digitally, or many ratios in an analog fashion.
The System
sRNA System
Boolean model for the sRNA system. This is mean to provide a significantly simplified mathematical understanding of the dynamics involved.
clear all clc %Constants and Values trackingNum = 6; %mRNA_pA, mRNA_pB, sRNA_A, sRNA_B, protein_A, protein_B; inputNum = 2; %A, B; processNum = 6; %Trx_pA, Trx_sRNA_A, Trx_pB, Trx_sRNA_B, Trl_A, Trl_B; %Cell/Matrix Dimensions m = 2^trackingNum; n = 2^inputNum; c = cell(m+1, n+1); trackingMatrix = de2bi(0:m-1); inputMatrix = de2bi(0:n-1); processMatrix = zeros(1,processNum); %Populating the Cell (Tracking x Input) c{1,1} = [0]; for j = 1 for i = 2:m+1 c{i,j} = trackingMatrix(i-1,:); end end for i = 1 for j = 2:n+1 c{i,j} = inputMatrix(j-1,:); end end for i = 2:m+1 for j = 2:n+1 c{i,j} = processMatrix; end end %Rules, Acessing, and Changing for j = 2:n+1 A = c{1,j}(1); B = c{1,j}(2); for i = 2:m+1 mRNA_pA = c{i,1}(1); mRNA_pB = c{i,1}(2); sRNA_A = c{i,1}(3); sRNA_B = c{i,1}(4); protein_A = c{i,1}(5); protein_B = c{i,1}(6); %Trx_pA if A == 1 c{i,j}(1) = 1; end %Trx_pB if B == 1 c{i,j}(2) = 1; end %Trx_sRNA_A if A == 1 c{i,j}(3) = 1; end %Trx_sRNA_B if B == 1 c{i,j}(4) = 1; end %Trl_A if mRNA_pA == 1 && mRNA_pB == 0 c{i,j}(5) = 1; end %Trl_B if mRNA_pB == 1 && mRNA_pA == 0 c{i,j}(6) = 1; end end end %Display Results (Column by Column) [nrows,ncols]= size(c); %Condense Cell and Display for i = 1:nrows for j = 1:ncols string = num2str(c{i,j}); l = length(string); r = 1; s = 1; t = 0; while t ~= 1 if r == l t = 1; end noSpacesString(s) = string(r); r = r+3; s = s+1; end c{i,j} = noSpacesString; end end c(:,:) %Find Steady States and Corresponding Inputs counter = 1; for i = 2:m+1 for j = 2:n+1 if c{i,j} == c{i,1} completeMatrix{counter,1} = c{i,j}; %Steady state values completeMatrix{counter,2} = num2str([i-1,j-1]); %Location (n x m) within results area completeMatrix{counter,3} = c{1,j}; %Corresponding input values counter = counter+1; end end end SS_mXn_Input = completeMatrix time = 0:processNum; %plot(time,,time,,time,,time,,time,,time,) %New Cell for Specific Cases % d(:,1) = c(:,1); % for j = 2:n+1 % C1 = '0000'; % C2 = '0011'; % C3 = '0100'; % C4 = '0111'; % C5 = '1000'; % C6 = '1011'; % C7 = '1100'; % % a = {C1, C2, C3, C4, C5, C6, C7}; % for b = 1:1:length(a) % if strcmp(c(1,j),a(b)) == 1 % d(:,b+1) = c(:,j); % end % end % end % d
ans =
'0' '00' '01' '10' '11'
'000000' '000000' '010100' '101000' '111100'
'000001' '000000' '010100' '101000' '111100'
'000010' '000000' '010100' '101000' '111100'
'000011' '000000' '010100' '101000' '111100'
'000100' '000000' '010100' '101000' '111100'
'000101' '000000' '010100' '101000' '111100'
'000110' '000000' '010100' '101000' '111100'
'000111' '000000' '010100' '101000' '111100'
'001000' '000000' '010100' '101000' '111100'
'001001' '000000' '010100' '101000' '111100'
'001010' '000000' '010100' '101000' '111100'
'001011' '000000' '010100' '101000' '111100'
'001100' '000000' '010100' '101000' '111100'
'001101' '000000' '010100' '101000' '111100'
'001110' '000000' '010100' '101000' '111100'
'001111' '000000' '010100' '101000' '111100'
'010000' '000001' '010101' '101001' '111101'
'010001' '000001' '010101' '101001' '111101'
'010010' '000001' '010101' '101001' '111101'
'010011' '000001' '010101' '101001' '111101'
'010100' '000001' '010101' '101001' '111101'
'010101' '000001' '010101' '101001' '111101'
'010110' '000001' '010101' '101001' '111101'
'010111' '000001' '010101' '101001' '111101'
'011000' '000001' '010101' '101001' '111101'
'011001' '000001' '010101' '101001' '111101'
'011010' '000001' '010101' '101001' '111101'
'011011' '000001' '010101' '101001' '111101'
'011100' '000001' '010101' '101001' '111101'
'011101' '000001' '010101' '101001' '111101'
'011110' '000001' '010101' '101001' '111101'
'011111' '000001' '010101' '101001' '111101'
'100000' '000010' '010110' '101010' '111110'
'100001' '000010' '010110' '101010' '111110'
'100010' '000010' '010110' '101010' '111110'
'100011' '000010' '010110' '101010' '111110'
'100100' '000010' '010110' '101010' '111110'
'100101' '000010' '010110' '101010' '111110'
'100110' '000010' '010110' '101010' '111110'
'100111' '000010' '010110' '101010' '111110'
'101000' '000010' '010110' '101010' '111110'
'101001' '000010' '010110' '101010' '111110'
'101010' '000010' '010110' '101010' '111110'
'101011' '000010' '010110' '101010' '111110'
'101100' '000010' '010110' '101010' '111110'
'101101' '000010' '010110' '101010' '111110'
'101110' '000010' '010110' '101010' '111110'
'101111' '000010' '010110' '101010' '111110'
'110000' '000000' '010100' '101000' '111100'
'110001' '000000' '010100' '101000' '111100'
'110010' '000000' '010100' '101000' '111100'
'110011' '000000' '010100' '101000' '111100'
'110100' '000000' '010100' '101000' '111100'
'110101' '000000' '010100' '101000' '111100'
'110110' '000000' '010100' '101000' '111100'
'110111' '000000' '010100' '101000' '111100'
'111000' '000000' '010100' '101000' '111100'
'111001' '000000' '010100' '101000' '111100'
'111010' '000000' '010100' '101000' '111100'
'111011' '000000' '010100' '101000' '111100'
'111100' '000000' '010100' '101000' '111100'
'111101' '000000' '010100' '101000' '111100'
'111110' '000000' '010100' '101000' '111100'
'111111' '000000' '010100' '101000' '111100'
SS_mXn_Input =
'000000' '1 1' '00'
'010101' '22 2' '01'
'101010' '43 3' '10'
'111100' '61 4' '11'
Kinase/Phosphatase System
Throughout this derivation, we borrow
For an opposing pair of enzymes modifying and unmodifying a substrate,
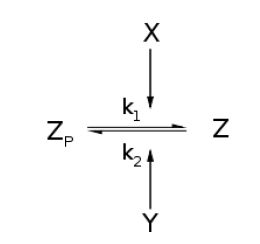
From Michaelis-Menten kinetics we know that the rate at which Zp is dephosphorylated is 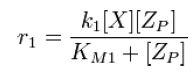
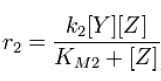
Along with the conservation equation [Z]o = [Zp] + [Z], File:1stblock.jpg Where File:2ndblock.jpg File:3rdblock.jpg Now, assume that the two opposing enzymes are nowhere near being saturated with substrate: File:4thblock.jpg So, the fraction of modified substrate is a saturating function of the ratio of inputs. The half-max value is the ratio of the strengths of the two enzymes. The linear regime of this function exists where File:5thblock.jpg And the fraction of unmodified substrate is a linear function of the ratio of inputs: File:6thblock.jpg So, the requirements for this analog ratio sensor are 1. Consistent relationships between inputs and enzyme activities (non-trivial) 2. A non-saturating amount of substrate 3. A ratio of enzyme activities greater than any ratios we want to measure Now increase the amount of substrate to saturation and File:7thblock.jpg File:8thblock.jpg modified. Any ratio above and all the substrate becomes unmodified. The requirements for this digital ratio sensor are: 1. Consistent relationships between inputs and enzyme activities (non-trivial) 2. An amount of substrate sufficient to saturate both opposing enzymes
References
[1] [http://www.ncbi.nlm.nih.gov/pubmed/6947258 Goldbeter A, Koshland DE Jr.: An amplified sensitivity arising from covalent modification in biological systems. PNAS. 1981 Nov;78(11)6840-4.]]
 "
"