Team:NYMU-Taipei/Experiments/Speedy switch
From 2010.igem.org
| Home | Project Overview | Speedy reporter | Speedy switch | Speedy protein degrader | Experiments and Parts | Applications | F.A.Q | About Us |
Contents |
Method
- Protocol:
1.Selected genes to be reported are incubated overnight in an LB liquid culture at 37oC and 180-200rpm. This makes sure they are fresh in the morning. Positive and negative controls are also needed.
2.Overnight liquid culture is diluted to OD600 of 0.1, Theophylline is added at concentrations ranging from 0.01mM to 20mM, and the mix incubated for 2-2.5 hours.
3.Measurement of OD at 2 hours: For each used well in the 96-well plate: Take 200uL from the liquid (make sure you pipette this step well) and put it in a cuvette to read the OD600. Note down the OD600 ["OD at 2 hours"], then take the liquid in the cuvette and put it in the right place in the 96-well plate.
4.Measurement of fluorescence: Continuous measurement of fluorescence with the excitation/emission wavelengths 488/511nm for 2 hours, with one fluorescence data point every 2 minutes.
5.Measurement of OD at 4 hours: For each used well in the 96-well plate: Take the liquid from the well and put it in the cuvette to measure the OD ["OD at 4 hours"].
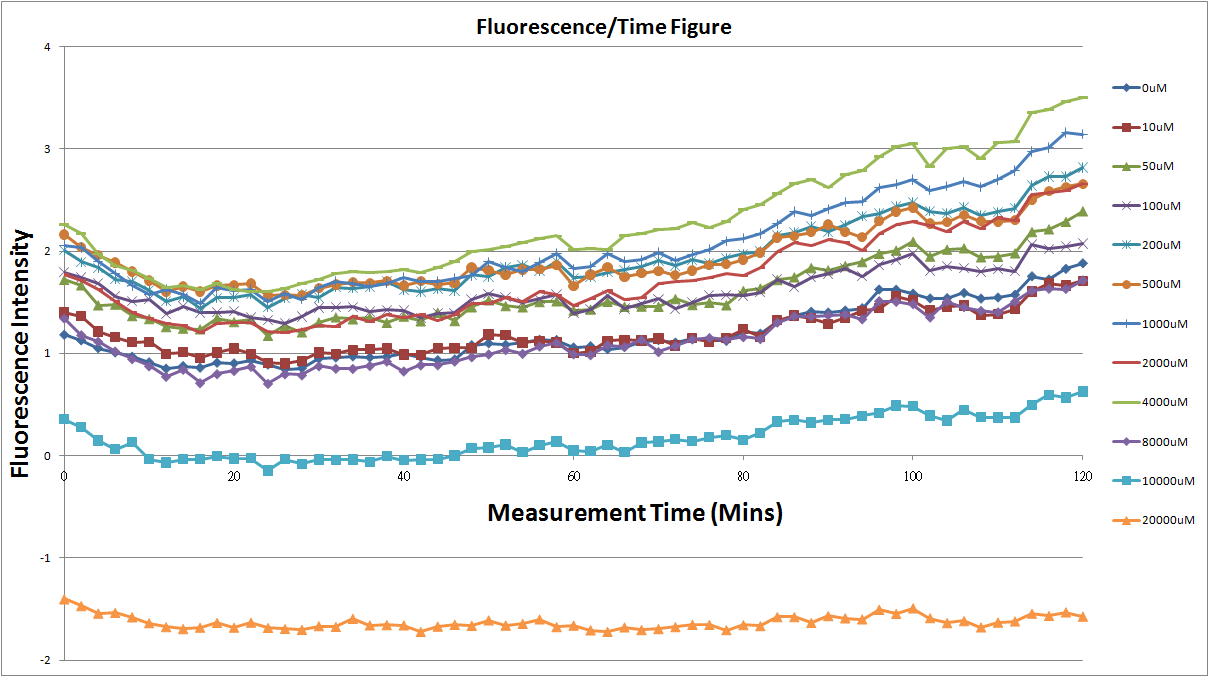
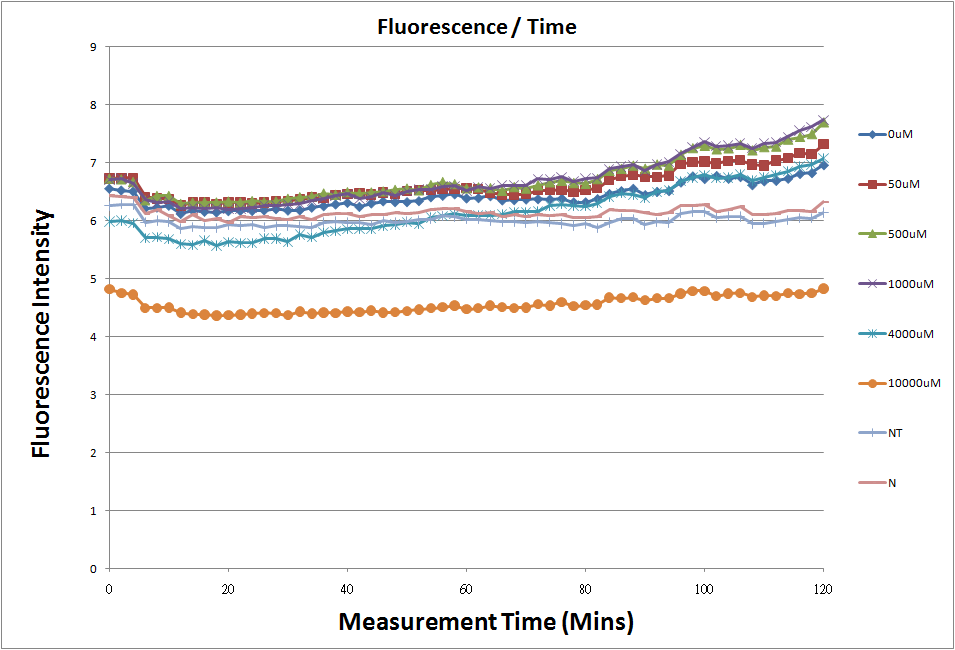
Reporting Assay1
- fig.1 is the original chart of the experiment.N line and NT line are control.N line stands for the sequence didn't have promoter.NT line stands for the sequence didn't have promoter but added 0.1μM theophylline.N line is compared with 0μM and it shows that even though the sequence didn't have promoter
- We test 12 different concentrations of theophylline to induce riboswitch.
Reporting Assay2
- We test 12 different concentrations of theophylline to induce riboswitch.
Reporting Assay3
- We test six different concentration of theophylline to induce riboswitch.
Repoting Assay4
- We test 6 different concentration of theophylline to induce riboswitch.
 "
"