Construction usu.html
From 2010.igem.org
Construction
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Pellentesque vel sapien lectus, in dignissim leo. Duis diam tortor, facilisis id tincidunt in, fringilla a est. Ut elit libero, venenatis porttitor ultrices eget, rutrum sed diam. Duis semper libero posuere lorem pharetra pretium. Nulla a lacus eu ante elementum rutrum et a neque. Aenean volutpat tellus non est tempus rhoncus in nec lacus. Vivamus ac nibh at eros porta iaculis. Duis non magna ligula. Vivamus nec ipsum quis magna faucibus eleifend pretium sed lectus. Pellentesque sit amet augue non dolor tempor pharetra. Vivamus rhoncus turpis sed risus mollis sit amet ullamcorper tellus molestie. Vivamus eget lorem eget purus scelerisque sollicitudin vel ut ipsum. Nam aliquet urna odio. Class aptent taciti sociosqu ad litora torquent per conubia nostra, per inceptos himenaeos. Etiam semper condimentum felis, eget mollis neque pulvinar et. Cras a augue enim, a facilisis nisi. Suspendisse potenti. Sed sit amet est augue. Quisque non tortor enim, quis pretium ante.
In addition to our work developing the cyanobacterial toolkit, an effort was made to complete and further characterize portions of our 2009 project [1] during this iGEM season. USU’s 2009 iGEM team focused on simplifying cellular product recovery by developing a platform for protein secretion systems in the BioBricks format.
Overview
Recovery costs of cellular products can account for as much as 80% of production expenses (Hearn and Acosta, 2001). In an attempt to reduce recovery costs, we developed a library of Silver fusion compatible gene constructs coding for secretion signal peptides. Five signal peptides commonly utilized in E. coli were converted to BioBrick format. Testing was performed by fusing signal peptides to the cycle 3 mutant of green fluorescent protein and the protein phasin. GFP was targeted as a model protein because of its ease of detection. Phasin is a protein coexpressed in organisms that produce polyhydroxyalkanoates (PHAs), a class of bioplastics. Phasin has been shown to interact and bind to PHA granules and to modify granule size (York, 2001; Maehara, 1999). Phasin targeted for secretion could produce a phasin-PHA complex, effectively transporting PHAs into the extracellular media. Transport of PHA into the media could reduce costs of bioplastic production and make this alternative plastic a more viable competitor to traditional petrochemically derived plastics. At the time of last year’s jamboree, SDS-PAGE indicated that GFP and Phasin were both successfully released into the extracellular media when fused to the GeneIII signal peptide.
New Contributions
Two additional secretion composite parts have been completed and submitted to the parts registry. These parts allowed us to test the functionality of the HlyA signal peptide. Most significantly, the proof of concept of the phasin/PHA complex secretion was verified using the HlyA targeted phasin.
Fig 1: Parts BBa_K390501 and BBa_K390502 submitted to the parts registry.
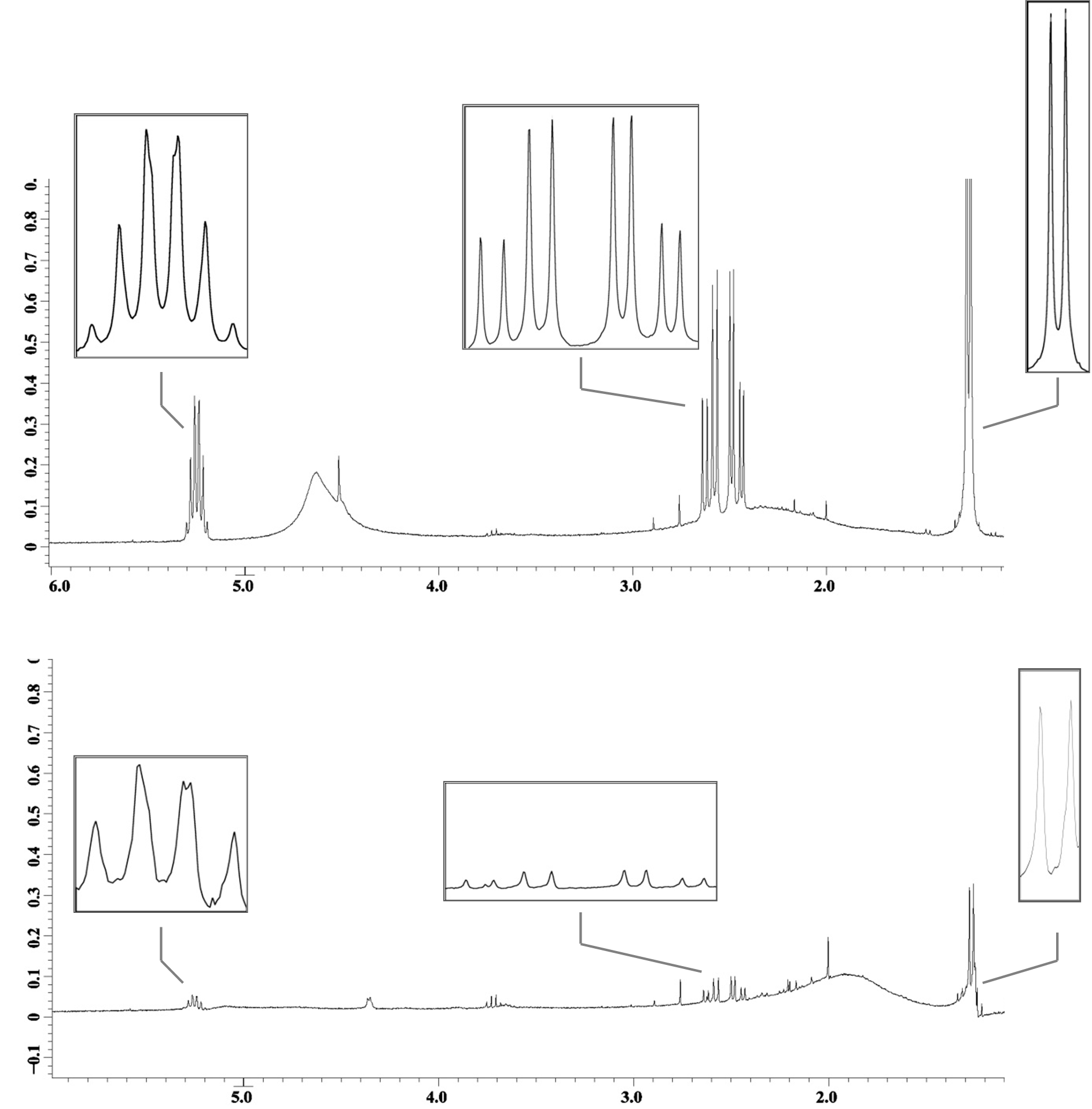
Cells containing the genes necessary for PHA production, HlyBD membrane proteins, and the HlyA targeted phasin (BBa_K390501) were grown in M9 minimal media. Cells containing the genes for PHA production and the HlyBD membrane proteins were grown as a negative control. After 30 hours of growth, a nucleating agent was added to both cultures and centrifuged. The resulting pellet was purified and analyzed using quantitative 1H NMR. Differences in intensity of the 1H NMR spectra indicate that PHA is released into the extracellular media when HlyA targeted phasin is expressed. We have reason to believe that this platform can be optimized to significantly reduce costs associated with commercial PHA production. This additional work further validates the parts created by USU’s 2009 team and the potential for their implementation in other cellular product recovery.
Fig 2: 1H NMR spectra with PHA characteristic peaks amplified. The top spectrum reports PHA isolated gravimetrically from a culture containing HlyA targeted phasin. The bottom spectrum reports PHA in the negative control. Notice the differences in intensity. Due to the similarity in density of PHA granules and E. coli, PHA in the negative control is expected as cells containing intracellular PHA can be present in the resulting pellet.
References
Hearn MT, Acosta D (2001) Applications of novel affinity cassette methods: Use of peptide fusion handles for the purification of recombinant proteins. J Mol Recognit 14:323-369
Maehara A, Ueda S, Nakano H, Yamane T (1999) Analyses of a polyhydroxyalkanoic acid granule-associated 16-kilodalton protein and its putative regulator in the pha locus of Paracoccus denitrificans. J Bacteriol 191:2914-2921
York GM, Junker BH, Stubbe J, Sinskey AJ (2001) Accumulation of the PhaP Phasin of Ralstonia eutropha is dependent on production of polyhydroxybutyrate in cells. J Bacteriol 183:4217-4226
 "
"