Team:Mexico-UNAM-CINVESTAV/Notebook/Week Four
From 2010.igem.org
Week #4
27th September - 01 October 2010
Monday
Well...we are stuck with the vector this week we have to get vector is our main objetive.
- We have achived a nanodrop readings as below
| ng/μl | 260/280 | 260/230 | |
| PsB1C3 | 0.60 | -0.73 | -0.0 |
| PCR 1 red | 427.70 | 1.71 | 1.71 |
| PCR 2 red | 483.20 | 1.74 | 1.71 |
| PCR 3 red | 410.70 | 1.76 | 2.02 |
| PCR 4 red | 431.05 | 1.61 | 1.88 |
| PCR 1 blue | 533.35 | 1.71 | 1.75 |
| PCR 2 blue | 536.00 | 1.72 | 1.82 |
| PCR 3 blue | 577.50 | 1.69 | 1.59 |
| PCR 4 blue | 627.55 | 1.70 | 1.56 |
| PsB1C3 | 4.10 | 2.62 | 1.01 |
- Yep our trouble is the lisis alcaline method to do the miniprep
we have a low concentration of vector’s plasmid.
We have to work an try to get more plasmid and make PCR's dilutions
is not posible to do ligations with this ratio between vector an insert.
On this step we advisor has proposed a method via Low Meelting agarose
to geting out plasmid from the band.
Tuesday
We run with a low meelting's agarose prove to extract band tomorrow
we do a protocol to precipit with alchol an salts our objetive is do it all
to get vector and do the ligations.
Wensday
Plan
- We prepared all to do ligations PsB1C3 with our Pcr's.
- For this we made a digest with EcoRI and PstI both vector and insert.
- We'll run a low melting gel slow 1 hour at 80 volts then purified from the band.
At Claudia's lab we cut tree bands from 2% agarose's gel of which two of
them where purified by Axigen kit the third one by a diferent kit.
The digestion was done with following amounts.
| DNA | 20μl |
| Buffer NB2 | 3μl |
| EcoRI | 2μl |
| PstI | 2μl |
| H2O | 2.4μl |
| BSA | 0.6μl |
| Total | 30μl |
Thursday
Plan
We going to proceed to do a dilutions and do the ligation
For this we do the following accounts
Poner las fórmulas usadas en un formato bonito
We did the ligations using the following amounts.
| Insert [20ng/μl] | 5μl |
| Vector | 1μl |
| Reaction buffer | 1μl |
| T4 DNA Ligase | 1μl |
| H2O | 2μl |
| Total | 10μl |
We used the follow notation.
- For vector purified from band: Pu
- For iGEM's vector (Vial with green cover): P
- Vector only digest: S
After one hour of ligation (time recomended by the protocol) we transformed by thermic shock
and left overnight plates to 37º
Friday
Today we discuss what we going to do with the AFP (anti freeze protein),
We going to transform by thermic shock plating on kanamicina medium then do
miniprep and cut with Xba and PstI we have to ligate at J13002 this as blackbone
the blackbone will cut with SpeI and PstI.
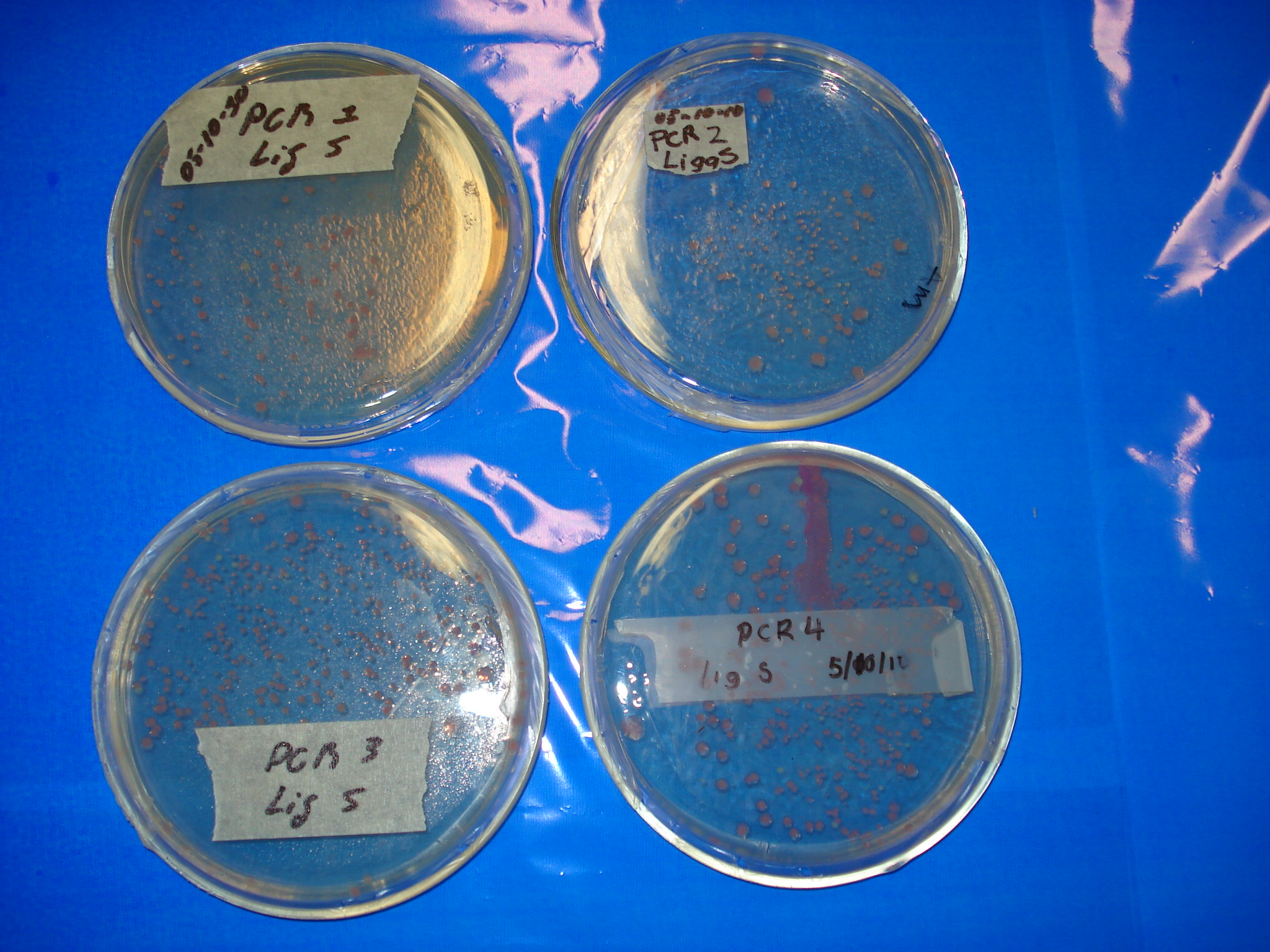
Meanwhile our ligations
- PCR1 Lig S
- PCR2 Lig S
- PCR3 Lig S
- PCR4 Lig Pu
- PCR4 Lig S
 "
"