Team:NYMU-Taipei/Project/Speedy switch
From 2010.igem.org
| Home | Project Overview | Speedy reporter | Speedy switch | Speedy protein degrader | Experiments and Parts | Applications | F.A.Q | About Us |
Contents |
Introduction
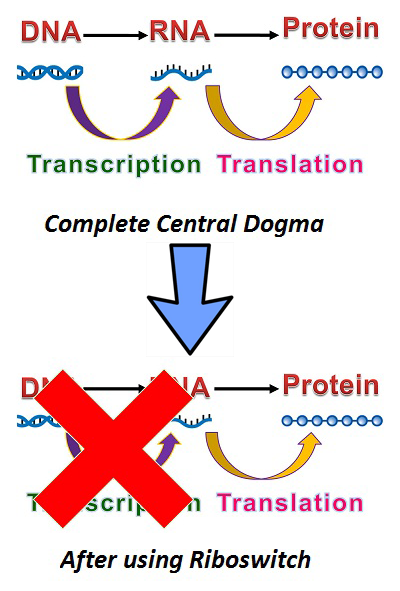
In the past, translating proteins from DNA has followed the central dogma of molecular biology: DNA to RNA to Protein. In theory, after transcription, ribosomes will bind to the ribosome binding site and begin translating mRNA into protein. During this process, we have no idea where the mRNA is; after the process, mRNA is quickly degraded. As a result, it is very hard to make a thorough inspection into mRNA's role in the central dogma. To solve this problem we need a mRNA level based switch which can be used to control its translation: a riboswitch.
By embedding the switch between the promoter and a gene, the gene expression will stop at the mRNA stage until a specific inducer is added and turn on the riboswitch. In other words, we can control when mRNA is translated.
Background
Before the discovery of RNA regulatory system , the only way to induce reaction in a cell was through inducible promoters. By turning these promoters on or off, we could control the transcription of the downstream DNA into RNA thus also controlling the translation of RNA to Protein. Yet even with these promoters, the regulation of several metabolic pathways was still unexplainable.
The discovery of the riboswitch was based on data which described conserved mRNA secondary structure found on 5’-untranslated regions and the creation of small-molecule binding mRNA, aptamers. The function of these riboswitches is similar to the function of inducible promoters in that they both regulate downstream genetic data: their difference is that while promoters regulate transcription of DNA, riboswitches control translation of mRNA.
A riboswitch is part of mRNA molecule that can bind a small molecule. When it does, the riboswitch will change its structure to regulate the following gene's activity. A riboswitch have two components: a sensor and an actuator. These two components work together to form a ‘switch’. The sensor binds to a small molecule inducer, and the actuator structurally changes to regulate gene expression. (Harbaugh et al.,2008)(Lynch et al., 2006)
Project
In our project, riboswitch is the critical switch between mRNA and protein. When a DNA is transcribed to an mRNA, riboswitch will stop central dogma at mRNA level. Then if we add inducer into riboswitch, the sensor will binds the inducer, and the actuator will act as a switch to turn on translation, and central dogma will go on.
Purpose
- Verify the protein function: we can perform RNA assay & protein assay in the same cell
- Control the protein expression
Design
We want to choose a kind of riboswitch which has the following characteristics:
- The inducer does not naturally exist or metabolize in the target organism.
- The riboswitch does not exist naturally in the target organism.
- The riboswitch does not have EcoRI, XbaI, SpeI, or PstI cutting sites.
- Having to modify a riboswitch with such cutting sites may cause more problems than it solves since the cutting site would have to be mutated and the secondary structure and molecule binding sites may change.
- Example of speedy switch: We found that the theophylline riboswitch fits all the requirements for use in Escherichia coli DH5α.
- What do we need for the project?
- We used Green fluorescent protein as our reporter for two main reasons. First, GFP makes a great reporter because it fluoresces when it activates, making it easy to detect. We can use the intensity of the fluorescence to measure the activity of the promoter. The second reason is that the GFP used is a biobrick, thus if another team needed to use this riboswitch circuit, it would be easy for them to attach another biobrick. So we chose biobrick - [http://partsregistry.org/Part:BBa_J04630 BBa_J04630] (GFP with terminator)
- Since most riboswitches already have a Ribosome binding site (RBS) in its structure, we did not add another RBS in front of downstream reporter.
- Our design:
- Promoter [http://partsregistry.org/Part:BBa_R0010 BBa_R0010]
- Riboswitch [http://partsregistry.org/Part:BBa_K411001 BBa_K411001]
- GFP+Terminator [http://partsregistry.org/Part:BBa_J04630 BBa_J04630]
Transform the whole structure,"promoter+ riboswitch+ GFP+ terminator in plasmid" to Escherichia coli. It will express GFP when theophylline (the inducer) in introduced
Experiment Design
To test our hyposthesis, we need to construct a circuit that has a promoter, a riboswitch, a reporter, and a terminator. We chose to use the theophylline riboswitch as it suited our requirements.
When the full sequence outlined above is transformed into the bacteria, it waits, inactivated, for the right small molecule inducer, in this case, theophylline. When theophylline is added, it will induce the riboswitch to fold differently to allow the translation of the downstream gene, without waiting for transcription.
By comparing the flourescence intensity data (the speed of GFP production), we can determine the difference in time between the traditional method of inducing promoters, to our method of inducing mRNA.
Since the sequence length of this riboswitch is relatively short, we decided to synthesize the riboswitch directly using two primers (which also contain the biobrick prefix and suffix):
- sequence
ggtgataccagcatcgtcttgatgcccttggcagcaccccgctgcaagacaacaag forward primer : gaattcgcggccgcttctagag ggtgataccagcatcgtcttgatgcccttggcag reverse primer : ctgcagcggccgctactagtacttgttgtcttgcagcggggtgctgccaagggcatcaagac
PCR expected result (99bp)
gaattcgcggccgcttctagagggtgataccagcatcgtcttgatgcccttggcag
gaattcgcggccgcttctagagggtgataccagcatcgtcttgatgcccttggcagcaccccgctgcaagacaacaagtactagtagcggccgctgcag
gtcttgatgcccttggcagcaccccgctgcaagacaacaagtactagtagcggccgctgcag
These two primers anneal with this common region.
- We then digested the riboswitch PCR product and a plasmid containing the plasmid backbone pSB1A2 with the restriction enzymes XbaI and PstI.
- After gel extraction/PCR purification of the relevant parts, we ligated them and produced the biobrick part [http://partsregistry.org/Part:BBa_K411101 BBa_K411101].
- Performed a back insert of [http://partsregistry.org/Part:BBa_J04630 BBa_J04630(GFP+terminator)] (digested with XbaI and PstI) into [http://partsregistry.org/Part:BBa_K411101 BBa_K411101] (digested with SpeI and PstI) and formed the biobrick [http://partsregistry.org/Part:BBa_K411102 BBa_K411102].
- Performed another back insert of [http://partsregistry.org/Part:BBa_K411102 BBa_K411102] (digested with XbaI and PstI) into [http://partsregistry.org/Part:BBa_R0010 BBa_R0010(lac promoter)] (digested with SpeI and PstI) and formed the biobrick [http://partsregistry.org/Part:BBa_K411103 BBa_K411103].
- Finally we test this kind of E. coli. We add Theophylline to induce riboswitch on and the GFP express the fluorescence.
- Finish the reporting assay, and we can have the plots of the fluorescence intensity and the theophylline quantity or the activity of the promoter.
Result
Reference
- [Svetlana V. Harbaugh et al.,08] Svetlana V. Harbaugh.et al. Riboswitch-based sensor in low optical background. Proc. of SPIE Vol. 7040
- [Lynch06] Sean A. Lynch, Shawn K. Desai, Hari Krishna Sajja, and Justin P. Gallivan1 (2006). A High-Throughput Screen for Synthetic Riboswitches Reveals Mechanistic Insights into Their Function. CELL DOI 10.1016/j.chembiol.
- [Topp07] Shana Topp and Justin P. Gallivan(2007)Guiding Bacteria with Small Molecules and RNA. JACS
- [Mandal03] Maumita Mandal, Benjamin Boese,Jeffrey E. Barrick, Wade C. Winkler, and Ronald R. Breaker1(2003). Riboswitches Control Fundamental Biochemical Pathways in Bacillus subtilis and Other Bacteria. Cell, Vol. 113, 577–586
 "
"