|
|
|
JUNE: WEEK 4
June, 21st
Planning of the activity of the week.
Freezer cleaning: for each ligation, we choosed the correct clone and stored it in the iGEM 2010 ligations box. All these cloned were gel screened and sequenced and re correct!
The colonies we choosed are:
| colony choosed |
ligation name |
| I0-2 | I0 |
| I1-2 | I1 |
| I2-1 | I2 |
| I3-1 | I3 |
| I4-2 | I4 |
| I5-1 | I5 |
| I6-2 | I6 |
Other colonies resulted psitives at the screening but were NOT sequenced and are stored in the iGEM 2010 cemetery box. These clones are: I1-1, I2-2, I4-1 and I6-3.
June, 22nd
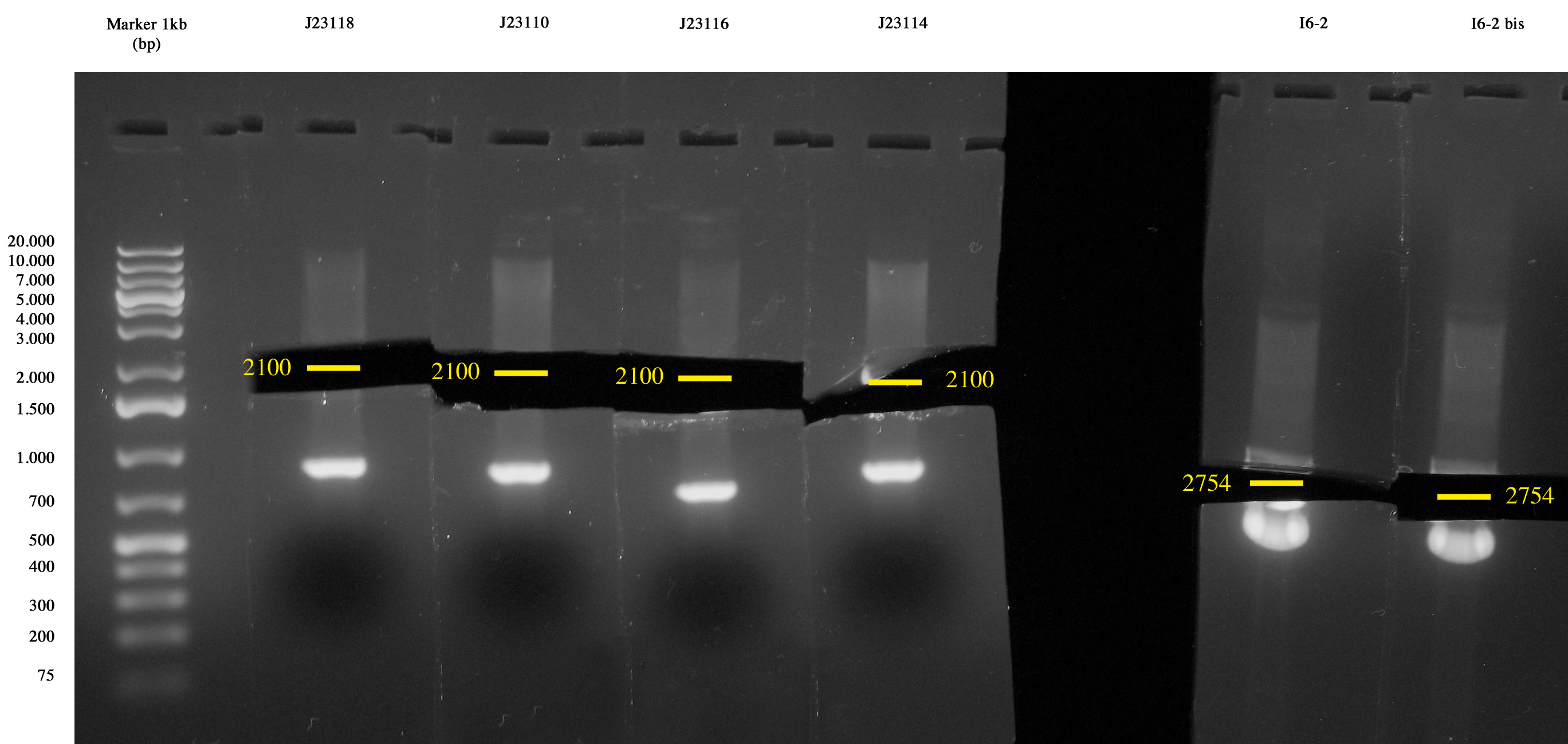
Inoculum of I6, <partinfo>BBa_J23118</partinfo>, <partinfo>BBa_J23110</partinfo>, <partinfo>BBa_J23114</partinfo>, <partinfo>BBa_J23116</partinfo> from glycerol stock in 5ml LB+Amp. Cultures were grown ON at 37°C 220rpm.
June, 23rd
Cultures incubated ON were all grown. Plasmids were extracted with MiniPrep.
After MiniPrep, purified DNA was quantified with NanoDrop.
| I6 | 193 ng/ul
|
| <partinfo>BBa_J23118</partinfo> | 107,9 ng/ul
|
| <partinfo>BBa_J23110</partinfo> | 83 ng/ul
|
| <partinfo>BBa_J23114</partinfo> | 89,8 ng/ul
|
| <partinfo>BBa_J23116</partinfo> | 82,7 ng/ul
|
|
Digestion of:
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 | Enzyme 2 | Buffer H
|
| I6 | Insert | 25 | 9,3 | 11,2 | 1 XbaI | 1 PstI | 2,5
|
| I6-bis | Insert | 25 | 9,3 | 11,2 | 1 XbaI | 1 PstI | 2,5
|
| <partinfo>BBa_J23118</partinfo> | Vector | 25 | 9,3 | 11,2 | 1 SpeI | 1 PstI | 2,5
|
| <partinfo>BBa_J23110</partinfo> | Vector | 25 | 12 | 8,5 | 1 SpeI | 1 PstI | 2,5
|
| <partinfo>BBa_J23114</partinfo> | Vector | 25 | 11,2 | 9,3 | 1 SpeI | 1 PstI | 2,5
|
| <partinfo>BBa_J23116</partinfo> | Vector | 25 | 12,1 | 8,4 | 1 SpeI | 1 PstI | 2,5
|
Digestions were incubated at 37°C for 3 hours, gel run and gel-extracted.
Ligations were performed ON at 16°C:
- I7: <partinfo>BBa_J23118</partinfo> (S-P)+ I6 (X-P)
- I8: <partinfo>BBa_J23110</partinfo> (S-P)+ I6 (X-P)
- I9: <partinfo>BBa_J23114</partinfo> (S-P)+ I6 (X-P)
- I10: <partinfo>BBa_J23116</partinfo> (S-P)+ I6 (X-P)
NanoDrop quantifications were not reliable, so every ligation was performed with 3ul of vector, 2ul of insert, 3ul of ddH20, 1ul of T4 ligase and 1ul of T4 ligase buffer.
These four promoters from Andersone Promoters Collection were choosed for their strength, measured in Arbitrary Units:
| Part | RFP (a.u.)
|
| <partinfo>BBa_J23118</partinfo> | 1429
|
| <partinfo>BBa_J23110</partinfo> | 844
|
| <partinfo>BBa_J23114</partinfo> | 256
|
| <partinfo>BBa_J23116</partinfo> | 396
|
Soon quantitative tests will be performed to quantify each promoter's strength in terms of RPU.
Today we received 2 stabs from registry HQ for <partinfo>BBa_K208001</partinfo> (one for each registry loation of this part),the Silver-fusion compatible BioBrick part that codes for phasin. This part is in a pSB3K3 plasmid is in E. coli ??? . Cultures were straked on LB+Kan (50 ug/ml) agar plates. Cultures were named PhaP-1 and PhaP-2. Plates were incubated ON at 37°C.
June, 24th
June, 25th
 Screening of I7, I8, I9, I10
|
|
 "
"