Team:Berkeley/Results
From 2010.igem.org
- Home
- Project
- Parts
- Self-Lysis
- Vesicle-Buster
- Payload
- [http://partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2010&group=Berkeley Parts Submitted]
- Results
- Judging
- Clotho
- Human Practices
- Team Resources
- Who We Are
- Notebooks:
- [http://www.openwetware.org/wiki/Berk2010-Daniela Daniela's Notebook]
- [http://www.openwetware.org/wiki/Berk2010-Christoph Christoph's Notebook]
- [http://www.openwetware.org/wiki/Berk2010-Amy Amy's Notebook]
- [http://www.openwetware.org/wiki/Berk2010-Tahoura Tahoura's Notebook]
- [http://www.openwetware.org/wiki/Berk2010-Conor Conor's Notebook]
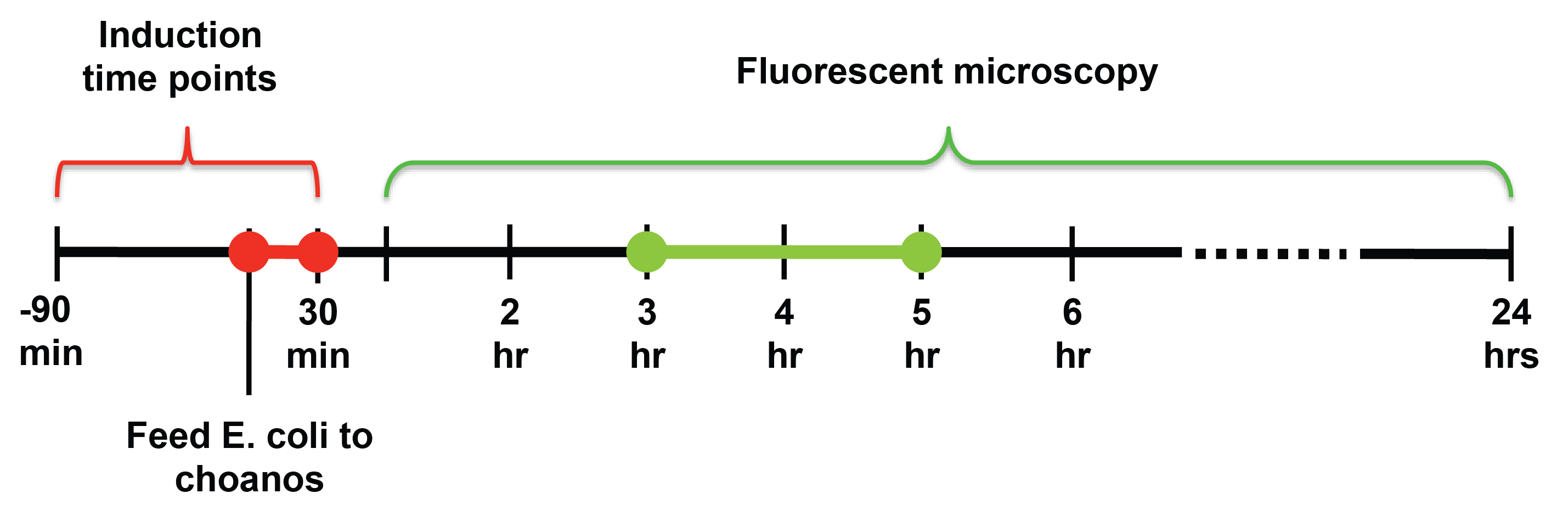
General Experimental Set Up
In general to assay payload delivery, we first
- Feed the payload bacteria to the choanos and induce self-lysis with arabinose. Induction of self lysis and feeding are not necessarily at the same time.
- Next, we assay for successful delivery events!
SInce the payload we used in our assays was GFP, we assayed for delivery events using fluorescent microscopy.
Challenges
One of the challenges we faced in assaying for payload delivery was finding a media that the choanos liked and that the payload bacteria were able to lyse in. After assaying several different media formulations, including CMG3, LB, TB, ASW and four different mixtures of ASW and LB, we found was that CMG3 media was the best media for choanos in which we were able to get substantial lysis.
The major challenge we faced with assaying for payload delivery was determining the timing of when to induce self-lysis and when to look at the choanos under the microscope.
For induction of self lysis, we tested timepoints from 90 minutes before feeding the choanos to 30 minutes after feeding the choanos, with 15 minute intervals. For looking at the choanos under the microscope, we tested timepoints from 1 hour after feeding to 24 hours after feeding.
From our experiments, we found that induction at the same time as feeding, or 15 or 30 minutes after feeding yielded the most delivery events. For timepoints before 15 minute before feeding, the number of delivery events drops off fairly steeply. SUPPORT WITH HEMOCYTOMETER DATA
We also observed that choanos seem to eat and digest bacteria with 1-2 hours after feeding, and that the best time to look for successful delivery was between 3 and 5 hours after feeding.
Controls
Our first step before assaying was to establish controls for all of our assay experiments. Based on how our parts should work, and work done in the Anderson Lab in mammalian cells, we expected that a sucessful delivery event would look like a choano who's cytoplasm was completely filled with GFP. With this in mind, the controls we ran regarding the choanos were:
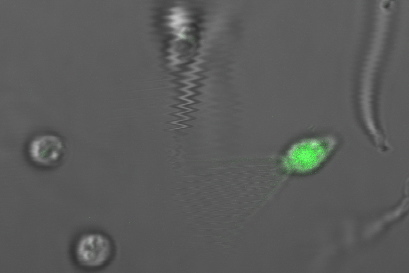
1.payload cells with no payload delivery device- This control was to establish a baseline of what normal eating and digestion of bacteria in choanos looks like. We observed that choanos seem to eat with 3-5 minutes after feeding. During the first hour or so, one generally sees bacteria in food vesicles , either as rod shapes or circles (rod shaped bacteria 'head on'). They are very bright and have signifcant halos. Slowly, we end up seeing fluorescence in small circular objects.The fluorescent intensity is less than the initial bacteria in food vesicles, and we believe flourescent payload is in the food vacuole. Eventually, the fluorescence disappears altogther, degraded by the vacuole of the choano.


(Left Image)Choanos feed just payload cells with no payload delivery device after 1 hour. You can see rod shaped bacteria in food vesicles in the choanos. (phase overlayed with green fluoresence, phase 50 ms, GFP 100ms) (Right Image) Choanos fed just payload cells with no payload delivery device after 3 hours . You can see a couple rod shaped bacteria inside of of choanos as well as many choanos with dimmer fluorescent circles, which we believe is gfp in the food vacuole.(phase overlayed with green fluoresence, phase 50 ms, GFP 300 ms.)
2.payload cells with uninduced payload delivery device'- This control was to ascertain that any delivery events we got were from induction with arabinose, and not just a normal phenotype exhibited in when choanos eat cells with self-lysis, vesicle buster and payload.
3.cells with payload and self lysis but no vesicle buster- This control was run to make sure that the successful delivery phenotype was saw was due to self-lysis device and the vesicle buster, not just self-lysis alone. We wanted to make sure that the what we were seeing was payload that had escaped the food vesicle, not just payload in a food vesicle.
4.cells with payload and vacuole buster, but no self-lysis - This control was once again to make sure that if we saw a successful delivery event, we could be certain that it was a phenotype unique to cells with induced self-lysis andvacuole buster.
Additionally, we considered controls related to fluorescent microscope settings. When imaging choanos, we kept in mind that with fluorescent microscopy, it would be easy to get misleading results if one is not careful to keep track of settings on the microscope, so we made sure to keep the exposure settings on the microscope fairly constant when running each assay.
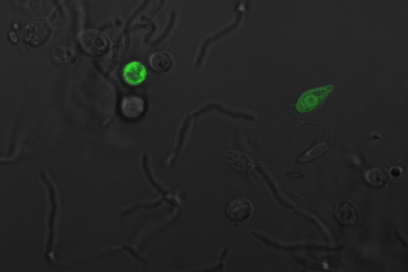
'Payload Delivery
The following images are of successful payload delivery events. As the images of the control above showed, the phenotype of GFP throughout the cytoplasm shown below is unique to constructs with self-lysis and vesicle buster, and is consistent with what we'd expect to see based on the biology of our parts.




Specific Protocols
or time points before feeding, we induce self lysis in a testube, and then put it in a 37 degree shaker until it is time to feed the choanos. For time points after feeding, we add arabinose to the choanos and keep them incubating at 37 C until we assay.
 "
"