Team:Stockholm/12 October 2010
From 2010.igem.org
Contents |
Nina
Gel clean up
I loaded samples in an agarose gel 1 % and ran at 80 V.
Samples:
- Protein A_TAT_N, _Tra10_N and _LMWP_N
- Fusion_CPP1, TAT_C ans _CPP3
- Fusion_TAT_N, _Tra10_N and _LMWP_N
The procedure was according to the description in protocols.
Weight of all samples: 200 mg
200 * 3 = 600 ul QXI
In step 3 I added 10 ul of QIAEXII to each sample.
Polyacrylamide gel
I ran a gel of the purified SOD.His (N terminal) to check if there had been any purification of the protein.
Ladder: PageRuler Unst. Protein Ladder
Arrangement on the gel:
Gel:
The gel was incubated in coomassie blue staining and destained on shake.
Unfortunately there were no bands representing SOD.His (~17 kDa) on the gel, which must mean that there was too small culture (12 ml) and that the cells have not been lysed properly. I will redo this work but with more start culture and lyse the cells in a more effiecient way.
The polyacrylamide gel was prepared by the SDS-PAGE mixtures described in protocols.
Andreas
Removal of insertion in BioBrick suffixes
Transformation results
Good colony yield on all plates. A few red colonies discovered on all plates, indicating that some partly digested pSB1C3 vector, still containing the RFP insert, had been excised during gel extraction.
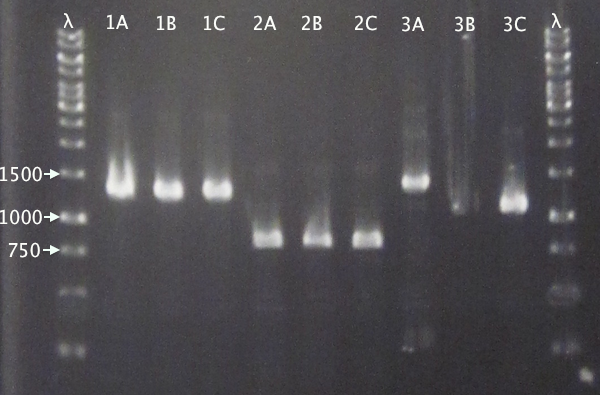
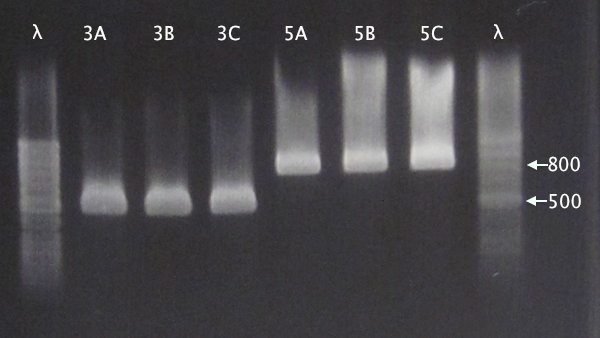
Colony PCR
From 11/10 transformations'
- pSB1C3.IgGp A-C
- pSB1C3.bFGF A-C
- pSB1C3.ProtA A-C
- pSB1C3.yCCS A-C
- pSB1C3.SOD A-C
- Standard colony PCR settings
- 1:15 elongation time
Gel verification
Gel 1: 1 % agarose, 140 V
Gel 2: 1 % agarose, 160 V
Expected bands
- pSB1C3.IgGp: 1262 bp
- pSB1C3.bFGF: 794 bp
- pSB1C3.ProtA: 506 bp
- pSB1C3.yCCS: 1076 bp
- pSB1C3.SOD: 791 bp
Results
- All clones correct.
- All clones correct.
- All clones correct.
- Correct size for clone C, weak band for clone B. Clone A probably carrying an RFP insert.
- All clones correct.
ON cultures
- 5 ml LB + Cm 25; 37 °C, 225 rpm
- 1C, 2C, 3C, 4C, 5C
BL21 transformation and growth test
Testing BL21 cells with/without CPP expression by transforming and inducing with IPTG.
- 50 μl 0.1 M IPTG
- 40 μl competent cells
- pEX.SOD⋅His
- pEX.nTra10⋅SOD⋅His
- pEX.nTAT⋅SOD⋅His
- pEX.nLMWP⋅SOD⋅His
Mimmi
SOD activity assay
- Kits?
- Biosite/trevigen 6000sek
- Biovision/labinova 3000sek, ca 1week delivery time
- Cayman
- Cellbiolabs
- Merch
- Abcam 3600sek
- Probior
- Sigma-Aldrich 3000sek
- T-cell technology inc.
- Kit from Sigma-Aldrich
- sponsored with a very good offer
 "
"