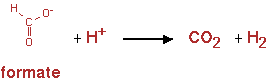Team:TU Delft/Modeling/MFA/additional pathways
From 2010.igem.org
Pathways added to the E. coli metabolic network
The E. coli network from Cell Net Analyzer contains, glycolysis, TCA cycle, pentose phosphate pathway, gluconeogenesis, anapleorotic routes, oxydative phosphorilization and biosynthesis pathways. CellNetAnalyzer can be found [http://www.mpi-magdeburg.mpg.de/projects/cna/cna.html here].
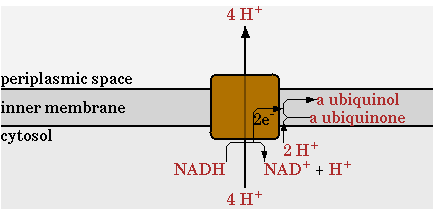
There was one change made in the network provided by CellNetAnalyzer for the NADH dehydrogenase reaction, annotated as NADHdehydro in CellNetAnalyzer. In the network of CellNetAnalyzer this reaction exports two protons, but this was changed to 4 as given by Ecocyc. This reaction is shown in figure 1.
Alkane degradation
To link alkanes to the existing network, the beta-oxidation was chosen as entry point. Several genese from biobricks were used to transform alkanes in to fatty acids which enter the beta oxydation cycle. These genes were:
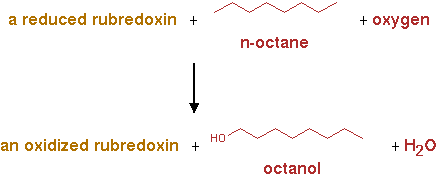
AlkB2 (EC 1.14.15.3)
The reaction for AlkB2 is shown in figure 2.
The reaction was implemented in CNA as;
n-alkane + reduced rubredoxin + O2 -> n-alkanol + oxidized rubredoxin
RubA3/RubA4 (EC 1.18.1.1)
The reaction for the regeneration of rubredoxin is shown in figure 3.
The reaction was implemented in CNA as;
oxidized rubredoxin + NADH -> reduced rubredoxin
ADH (EC 1.1.1.1)
The reaction for ADH is shown in figure 4.
The reaction was implemented in CNA as;
n-alkanol -> n-aldehyde + NADH
ALDH (EC 1.2.1.3)
The reaction for ALDH is shown in figure 5.
The reaction was implemented in CNA as;
n-aldehyde -> n-fatty acid + NADH
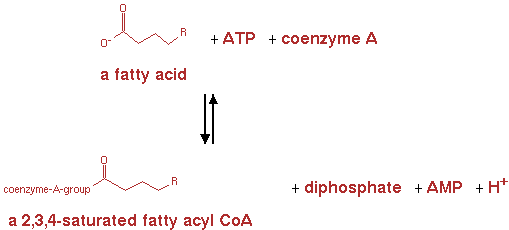
fatty acyl-CoA synthetase (EC 6.2.1.3)
From here the genes are already present in the E. coli genome. They were not yet present in the metabolic network for E. coli in CellNetAnalyzer however. So the following reactions and pathways were also implemented in CNA. The reaction for fatty acyl-CoA synthetase is shown in figure 6.
The reaction was implemented in CNA as;
n-fatty acid + ATP -> n-saturated fatty acyl-CoA + AMP
Adenylate kinase (EC 2.7.4.3)
CNA did not have a reaction to regenerate AMP yet, so the reaction for adenylate kinase was added to the network. The reaction for adenylate kinase is shown in figure 7.
The reaction was implemented in CNA as;
ATP + AMP -> 2 ADP
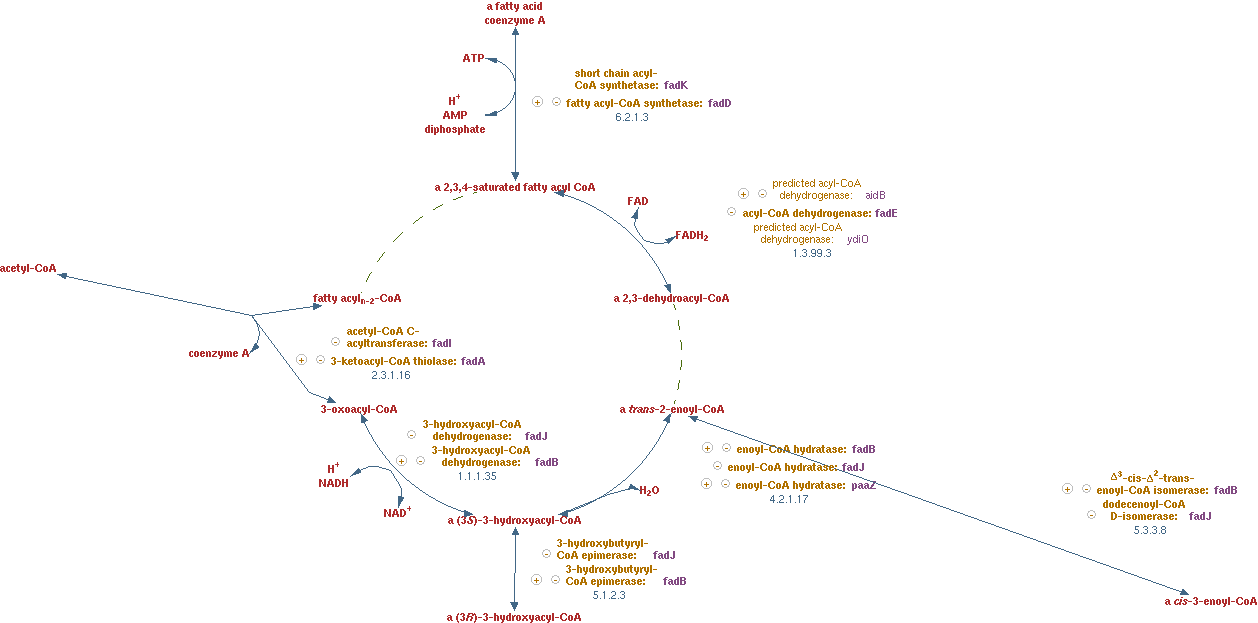
Fatty acid beta-oxidation cycle (EC 1.3.99.3 EC 4.2.1.17 EC 1.1.1.35 EC 2.3.1.16)
The reactions for this pathway are shown in figure 8.
The lumped reaction was implemented in CNA as;
n-saturated fatty acyl-CoA -> (n - 2)-saturated fatty acyl-CoA + acetyl-CoA + FADH2 + NADH
FADH2 regeneration
The cofactor FADH2 is not yet defined in the network of CNA, so a reaction had to be introduced to regenerate it. FADH2 gives its electrons to ubiquinol just like NADH, however in this process no protons are exported.
The reaction was implemented in CNA as;
1 FADH2 -> 1 QH2
Odd numbered alkanes
For all even numbered alkanes the above reactions completely link them to the main network in CNA. All the cofactors have regeneration reactions and all the alkanes are converted into acetyl-CoA. The final reaction in the beta oxidation cycle (n = 4) produces then two acetyl-CoA. For odd numbered alkanes the final reaction however (n = 5), a propionyl-CoA is generated;
n-saturated fatty acyl-CoA -> propionyl-CoA + acetyl-CoA + FADH2 + NADH
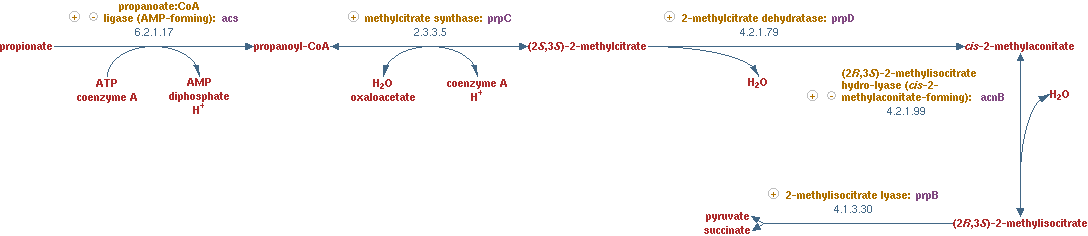
2-methylcitrate cycle (EC 2.3.3.5 EC 4.2.1.79 EC 4.2.1.99 EC 4.1.3.30)
This propionyl-CoA still needs to be linked to the main network in CNA. The pathway that was used to process this metabolite, was a part of the 2-methylcitrate cycle. The reactions for this pathway are shown in figure 9.
The lumped reaction was implemented in CNA as;
propionyl-CoA + oxaloacetic acid -> succinate + pyruvate
Biomass formation
The biomass is formed by many anabolic reactions that make monomers. All the anabolic reactions start at the so called key metabolites. There are 12 key metabolites and they are all in the glycolytic pathway and the TCA cycle. In the tool they are the red metabolites. For this analysis the anabolic reactions already present in CNA were used.
NO3 as electron acceptor (EC 1.7.99.4)
In oily environments oxygen diffuses more difficult into the water phase. The oxygen is used for the oxydative phosphorylation, regenerating NADH, and for the first step in the hydrocarbon degradation. To be more efficient with oxygen an additional electron acceptor was introduced.
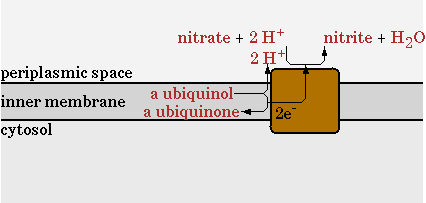
The standard oxydative phosphorylation (EC 1.6.5.3 EC 1.10.2.-) is shown in figure 10.
The second step will be disabled in the network and be replaced with a nitrate reductase. This reaction is shown in figure 11.
The reaction was implemented in CNA as;
NO3- + QH2 -> NO2-
This reaction uses NO3 as an electron acceptor to regenerate NADH and export protons to generate ATP. Less protons are exported per mol of NADH in comparison with oxygen as electron acceptor, so the ATP/NADH ratio will drop. The goal of implementing this pathway however, is to see how much the oxygen requirement of E. coli can maximally be reduced.
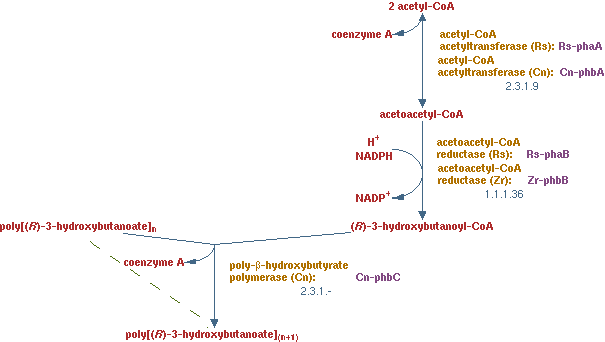
PHB production (EC 2.3.19 EC 1.1.1.36 EC 2.3.1.-)
In previous situations the hydrocarbons were degraded only to form biomass and CO2. It is interesting to see how much product could be made from hydrocarbons. PHB is a polymer of polyhydroxybutyrate. The production pathway of PHB is well known. PHB is a solid product which is easy to recover in the down stream process.
The pathway is displayed in figure 12.
The lumped reaction was implemented in CNA as;
2 acetyl-CoA + NADPH -> (R)-3-hydroxybutanoyl-CoA
the polymerization reaction just consumes (R)-3-hydroxybutanoyl-CoA, because a solid has no concentration in the liquid and therefore does not need to fulfill the steady-state condition. There are limiting factors to this reaction, but those are not considered in this analysis.
Hydrogen production
As with PHB, this pathway was added to the metabolic pathway to see how much product could be made from the alkane degraation in stead of just biomass and CO2 formation. Hydrogen is considered a green fuel. Hydrogen is a volatile product and is also easily separated from fermentation broth. It does however contain no carbon atoms, so hydrogen will still result in production of CO2 and biomass.
The pathway is shown in figure 14
The reaction was implemented in CNA as;
Formate -> H2 + CO2
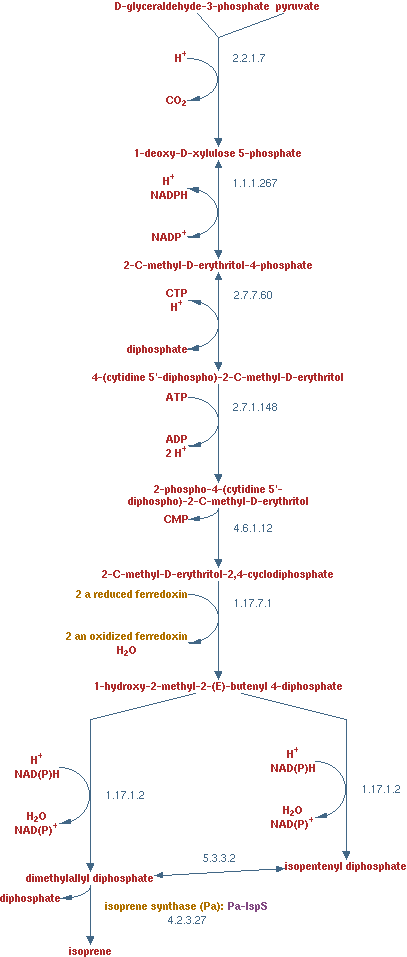
Isoprene production
In previous situations the hydrocarbons were degraded only to form biomass and CO2. It is interesting to see how much product could be made from hydrocarbons. Isoprene is a volatile product found in plants. E. coli will not be able to produce is in the near future, but it is an interesting product It is a very reduced product, with a similar amount of electron per carbon atom. Hydrocarbons have 6 - 6.3 electrons per carbon atom depending on the length and isoprene has 5.6 electron per carbon electron. If these values are close to each other, it has a positive influence on the maximal theoretical yield. Also the volatile nature of isoprene is very favorable for the downstream process
- note: make link open up a new window
The pathway is displayed here
In this scenario the lumped isoprene production pathway was added to metabolic network;
1 pyruvate + 1 D-glyceraldehyde-3-phosphate + 1 NADPH + 3 NADH + 3 ATP -> isoprene + CO2
isoprene export
 "
"