RBS digestion: Revenge
| Reagent
| Amount
|
| 1-2M
| 10 uL
|
| DW
| 4 uL
|
| 10x M Buffer
| 2 uL
|
| BSA
| 2 uL
|
| Xba I
| 1 uL
|
| Pst I
| 1 uL
|
| Total
| 20 uL
|
→Incubated at 37C for 60 min
- Added 4 uL of 6x Sample Buffer making it total of 12 uL per lane and electrophoresed
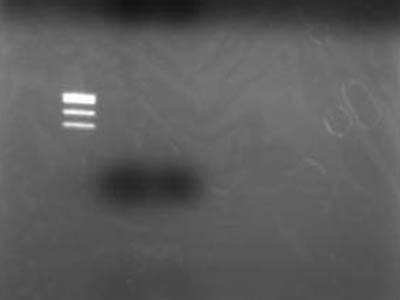
→Extracted for gel
→Electrophoresed 10 uL of Extracted DNA
Failure
- After better inspection of Kit specs it came to lite that retreavel rate for 50 bp and less is 26%
- RBS is just quite small when cut
Ligation
Preparation of DNA Solution for Ligation
| Part
| By comparison toLambda/Hind III (ng/10 uL)
| ng/uL
| ratio
| size (bp)
| (ng) required
| (uL) used
|
| Vector
| 50 ng/10 uL
| 5 ng/uL
| 1
| 2996 bp
| 10 ng
| 2 uL
|
| RFP
| 250 ng/10 uL
| 25 ng/uL
| 2
| 700 bp
| 7 ng
| 0.3 uL
|
| double terminator
| 5 ng/10 uL
| 0.5 ng/uL
| 2
| 200 bp
| 2 ng
| 4 uL
|
| Total
| 6.3 uL
|
Ligation and Transformation
| Reagent
| Amount
|
| DNA solution
| 6.3 uL
|
| Ligation solution
| 6.3 uL
|
| T4 ligase
| 1 uL
|
| Total
| 13.6 uL
|
- Incubated at 16C for 30 min
- Transformation: Added all to 50 uL of competent cell
- Incubated at 0C for 30 min
- Heat shocked at 42C for 60 sec
- 5 min on ice
- Added 100 uL of LB
- Incubated at 37C for 120 min
- Spread onto the LBC plate
- ncubated at 37C for 15~20 hrs
RBS 再リベンジ
Digestion
| Reagent
| Amount
|
| (RBS)1-2M
| 10 uL
|
| DW
| 4 uL
|
| 10x M Buffer
| 2 uL
|
| BSA
| 2 uL
|
| Xba I
| 1 uL
|
| Pst I
| 1 uL
|
| Total
| 20 uL
|
→37℃,60 min
エタ沈
- 2 uLの3 M 酢酸ナトリウムを加えた
- 44 uLのエタノールを加えた
- 液体窒素の中で凍らせた(1.5 mLのチューブに移す)
- 溶かしてから15,996 rpm @4℃で5分間遠心した
- 上清をほかのチューブに移した(一応これも遠心し,上清を捨て,同じ操作をした)
- 100 uLの70%エタノールで壁をリンスし,15,996 rpm @4℃で5分間遠心した
- 上清を捨て,真空デシケータで乾燥させた
- TE 5 uLで溶かした
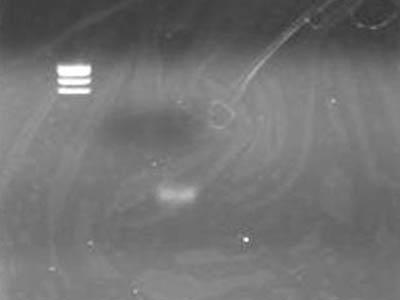
電気泳動
- TEで溶かしたDNA Solution 1 uLに6x SB 1 uLを加えた
- 最初にとった上清も同じ操作をした
| Lane
| DNA
|
| 2
| TSUDA Marker 1
|
| 3
| 上清
|
| 4
| DNA solution
|
 "
"