|
JULY: WEEK 5
July, 26th
July, 27th
July, 28th
July, 29th
We used our new synthesized primers to modify through PCR <partinfo>BBa_K208001</partinfo> phasin in order to create two new parts without stop codon, with Standard prefix and Silver suffix and Silver prefix and suffix.
 Gel run for PCR-modified phasins Gel extraction for phasins showed the following quantifications:
- Pha-10S-1: 15 ng/ul
- Pha-10S-2: 18,5 ng/ul
- Pha-SS-1: 22,8 ng/ul
- Pha-SS-2: 22,4 ng/ul
Phasins were digested X-S for 3 hours and were quantified:
- Pha-10S-1 (X-S): 14 ng/ul
- Pha-10S-2 (X-S): 13,2 ng/ul
- Pha-SS-1 (X-S): 15 ng/ul
- Pha-SS-2 (X-S): 14,9 ng/ul
Competentization of MC1061 (again) because we suspect the presence of a contaminant (it grew without reason on Cm plates).
Transformation of new MC1061 competent cells with:
- 1 ul (4ng) of miniprepped ENTERO-pSB4C5 (positive control);
- 1 ul of RING ligation (RING shouldn't be propagated in MC1061);
- 1 ul of MilliQ (negative control).
Transformed cells have been plated on Cm 12,5 ug/ml agar plates and incubated overnight at 37°C.
Tecan Test
July, 30th
We checked the presence of colonies in plates.
 MC1061 transformed with ENTERO-pSB4C5 (positive control) |  MC1061 transformed with RING |  MC1061 transformed with MilliQ (negative control) |
We calculated (thanks Federica) efficiency as #colonies/ug DNA plated
| Strain | Vector | #colonies | Efficiency
|
| MC1061 | ENTERO-pSB4C5 | 3700 | ~10^6
|
| MC1061 | RING | 0 | -
|
| MC1061 | NOTHING | 0 | -
|
As espected RING and NOTHING didn't grow.
MC1061 competent cells don't replicate RING :) !!!
Miniprep of J04450 to take the vector pSB1A3
3-hour digestion (X-S) and gel run to extract the backbone (~2157 bp).
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 (ul) | Enzyme 2 (ul) | Buffer H
|
| <partinfo>BBa_J04450</partinfo> | Vector | 25 | 17,2 | 3,3 | 1 X | 1 S | 2,5
|
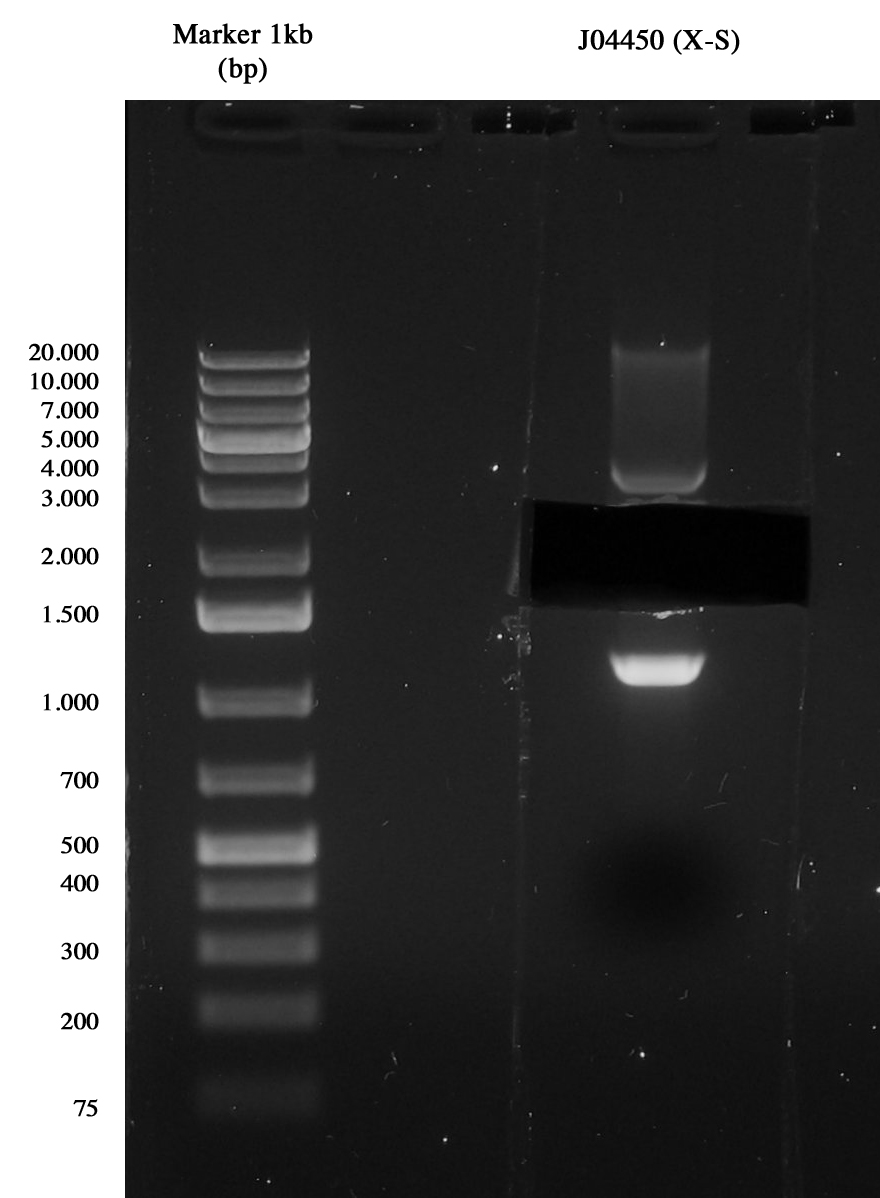 Gel run of J04450 to take pSB1A3 vector Gel extraction was quantified 15,5 ng/ul.
Ligation of:
- I20: Pha-10S-1 (X-S) + pSB1A3 (X-S)
- I21: Pha-SS-1 (X-S) + pSB1A3 (X-S)
July, 31st
Transformation of I20 and I21 into E. coli DH5-alpha. Cells were plated on LB+Amp agar plates and grown overnight at 37°C.
August, 1st
All plates showed colonies (there were also red colonies that contained a wrong ligated plasmid). So we picked three colonies each plate (the right ones) and inoculated them into 5 ml LB+Amp. They were let grow ON at 37°C, 220 rpm.
|
|