Team:Cambridge/Bioluminescence/Vibrio Characterisation
From 2010.igem.org

This page describes characterisation for part [http://partsregistry.org/Part:BBa_K325909 BBa K325909], the lux operon from Vibrio fischeri.
Description

This page described the lux operon from Vibrio fischeri. To relieve LuxR control we placed Lux C, D, A, B, E under the pBad promoter.
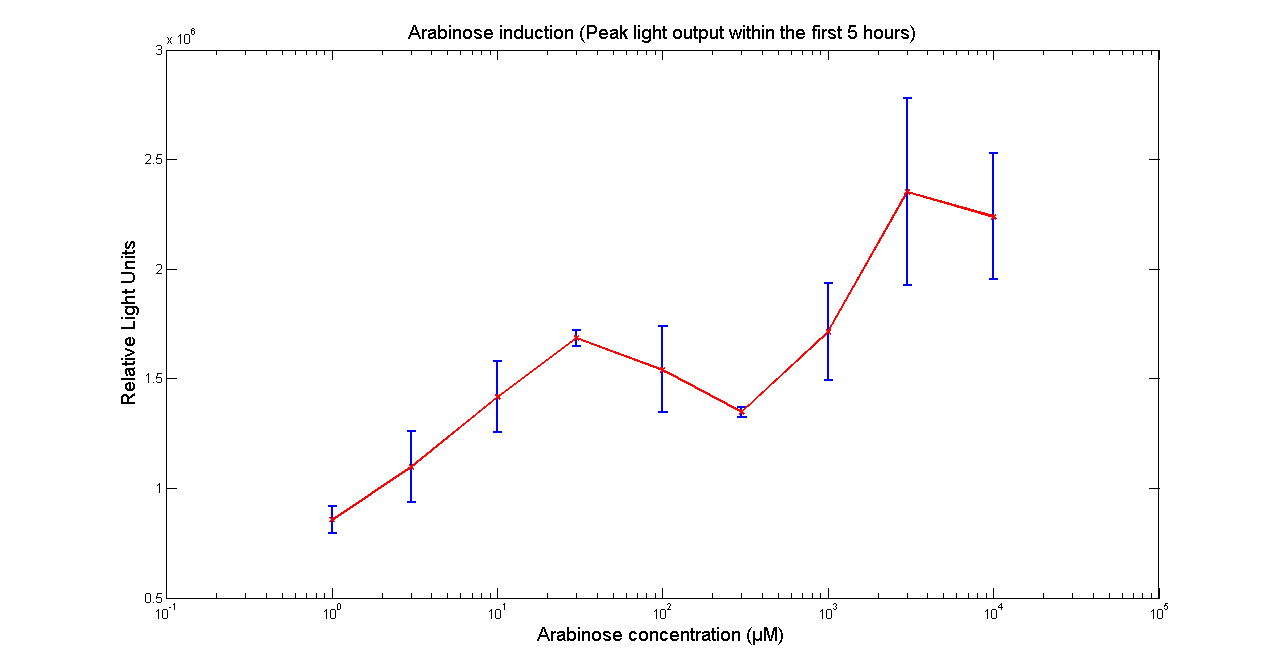
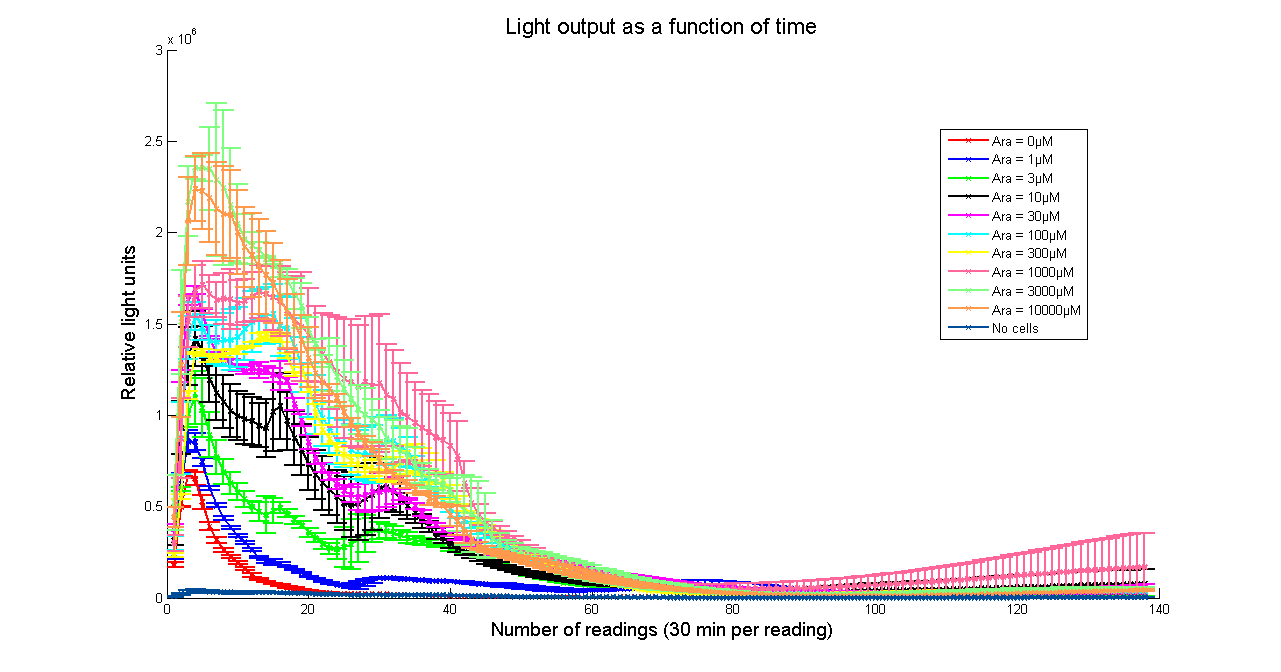
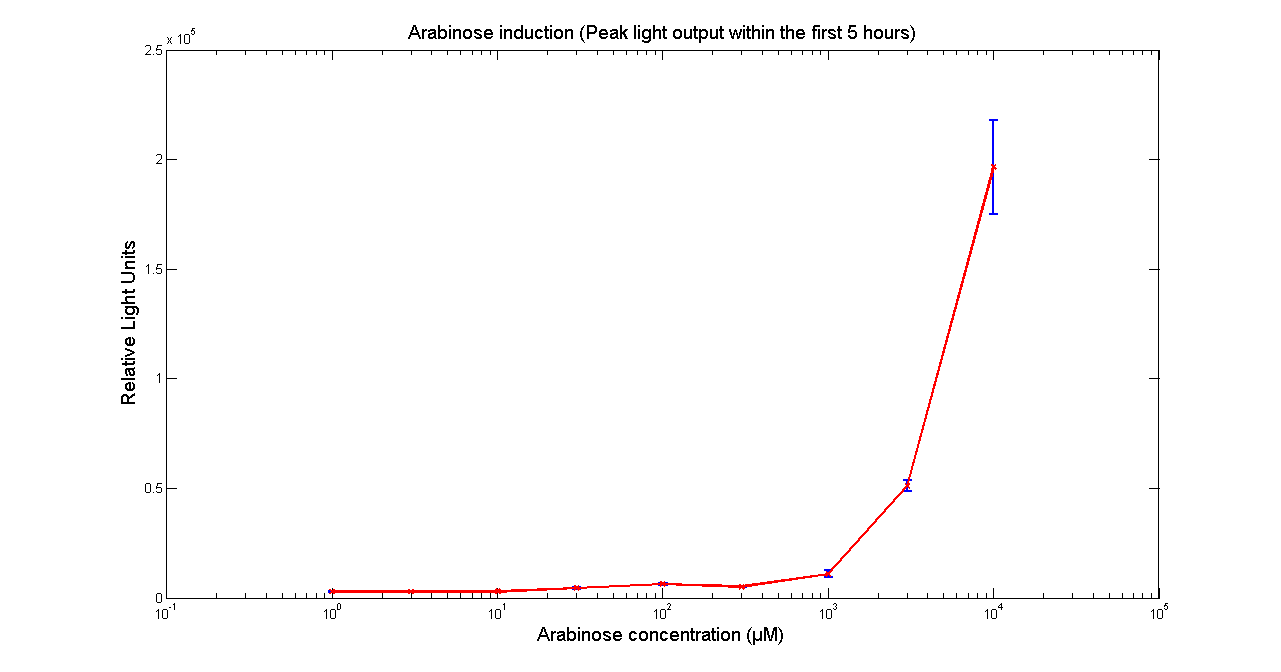
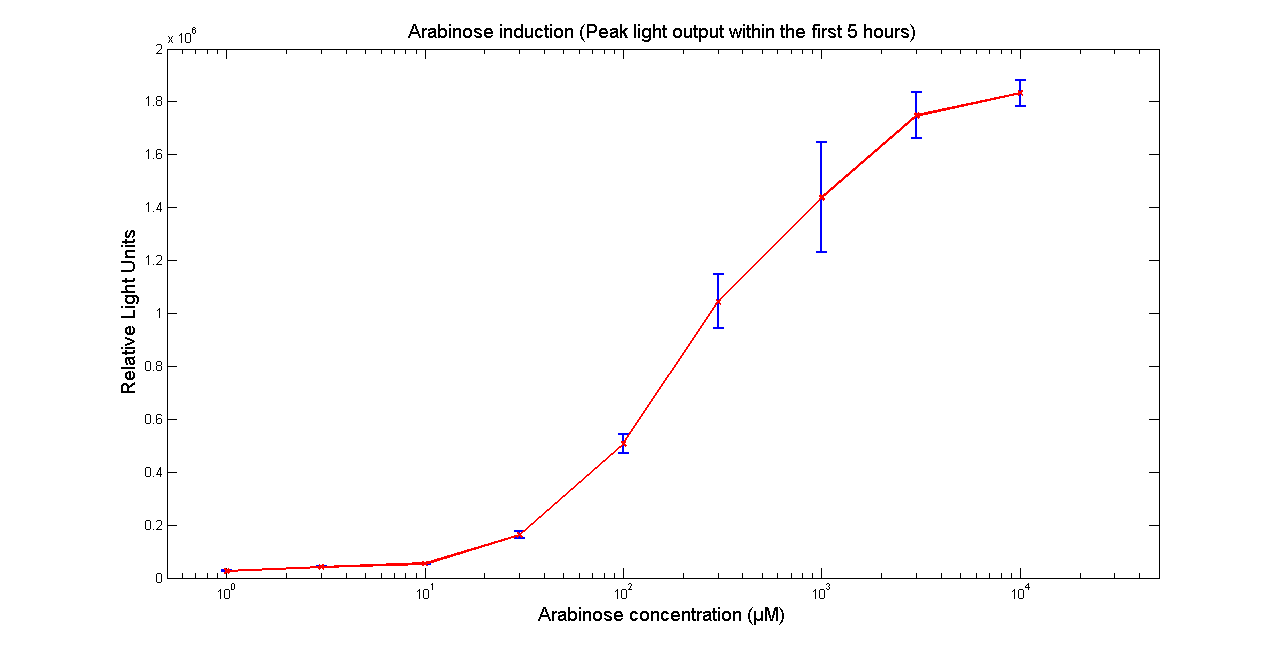
Arabinose to light
This page describes the relationship between Arabinose concentration in the medium with light output. We used a [http://www.bmglabtech.com/products/microplate-reader/instruments.cfm?product_id=2 FLUOstar OPTIMA] microplate reader to quantify the light output. Protocol and plate reader settings used are given below.
Data
| Data | Notes | Date Uploaded |
|---|---|---|
| Media:BBa_K325909ArabinosetoLight.xls | Raw data from experiment | 21/10/2010 |
H-NS mutants
It has been shown that the expression of the Vibrio fischeri lux operon when cloned into E. coli was repressed. This repression was linked to the nucleoid protein H-NS. To investigate this effect we cloned the operon into mutant E.coli cells in which the expression of the H-NS protein had been modified. We used a [http://www.bmglabtech.com/products/microplate-reader/instruments.cfm?product_id=2 FLUOstar OPTIMA] microplate reader to quantify the light output. Protocols and plate reader settings used are given below.
Data
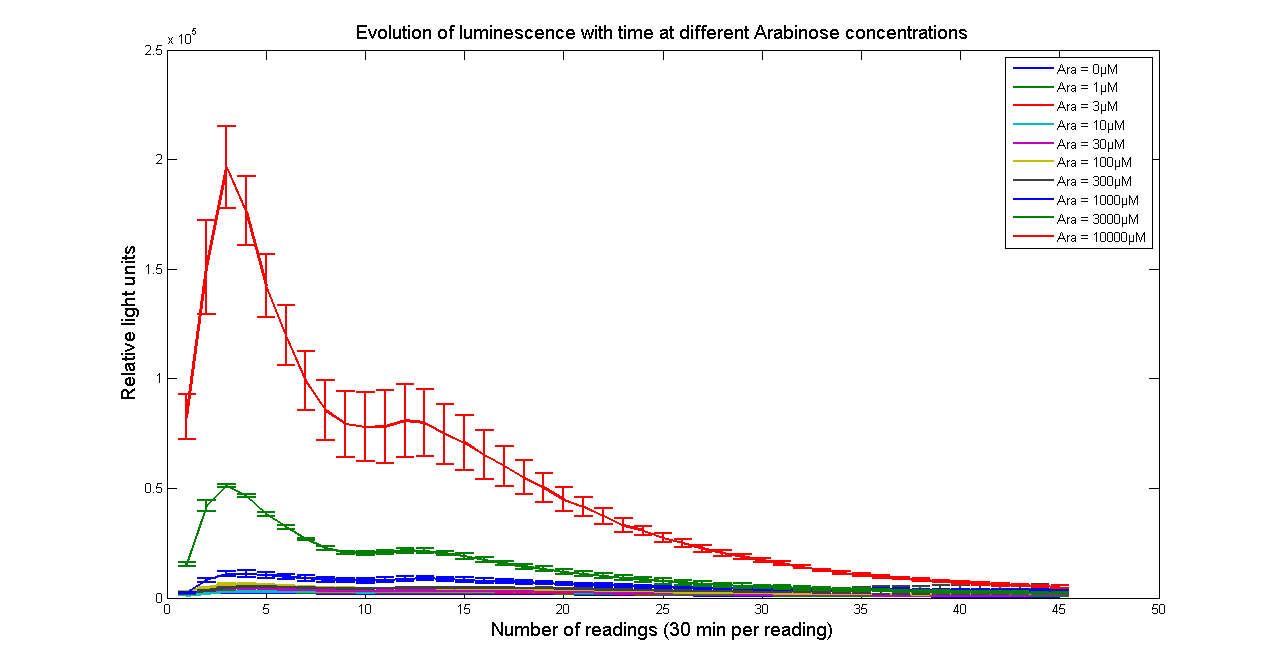
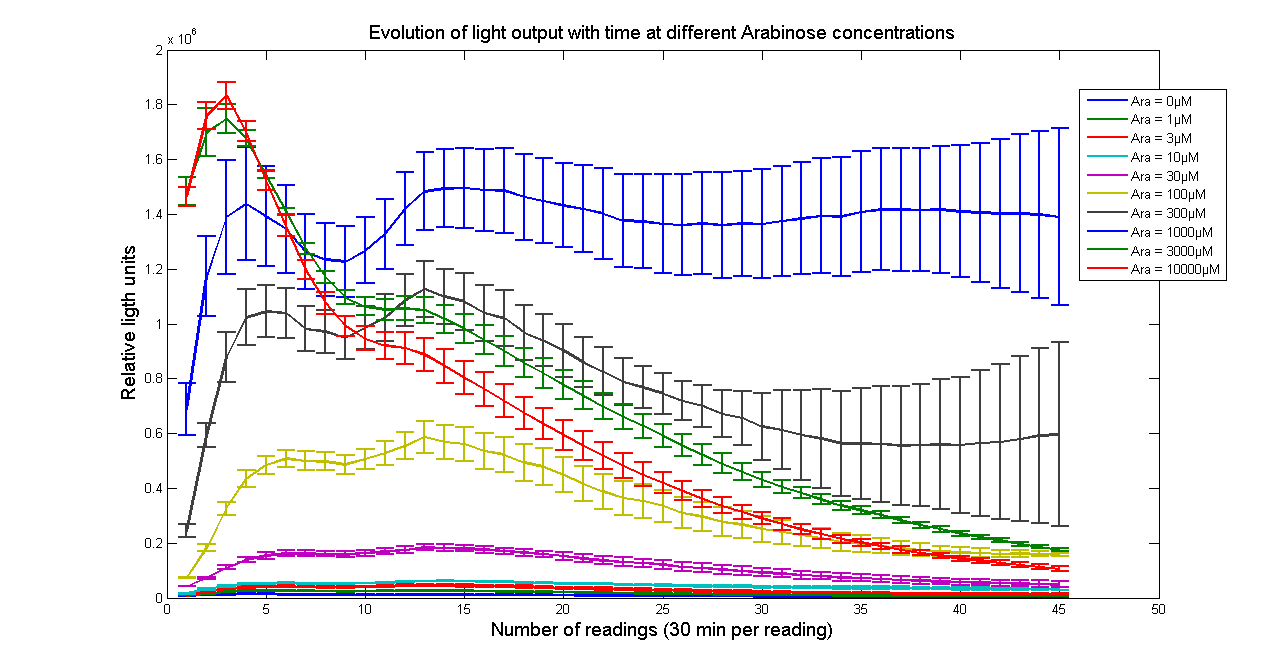
| Data | Notes | Date Uploaded |
|---|---|---|
| Media:BBa_K325909Mutants.xls | Raw data from experiment | 21/10/2010 |
Effect of pH
Both the intensity and the spectrum emitted by the luciferase-luciferin reaction has been shown to be higly dependent on the pH of the medium. The main characterisation experiments have been performed in LB Broth at pH 7, so in order to assess this effect cultures with LB and a citrate buffer were prepared (pH = 5.3, pH 6.1 and pH = 7) This page describes the results of these experiments. We used a [http://www.bmglabtech.com/products/microplate-reader/instruments.cfm?product_id=2 FLUOstar OPTIMA] microplate reader to quantify the light output. Protocols and plate reader settings used are given below.
Data
Maximum light output within 5 hours of D-luciferin injection at different pH values.
[http://partsregistry.org/wiki/images/e/e8/Phhistogram.png http://partsregistry.org/wiki/images/thumb/e/e8/Phhistogram.png/569px-Phhistogram.png]
These values are the mean of 3 readings. The corresponding error bars represent an interval of twice the standard deviation across the 3 data points centred around the mean value.
Evolution of light output at different values of pH.
[http://partsregistry.org/wiki/images/d/d8/Phtimecourse.png http://partsregistry.org/wiki/images/thumb/d/d8/Phtimecourse.png/569px-Phtimecourse.png]
Measurements are taken every 20 min. These values are the mean of 3 readings. The corresponding error bars represent an interval of twice the standard deviation across the 3 data points centred around the mean value.
| Data | Notes | Date Uploaded |
|---|---|---|
| [http://partsregistry.org/wiki/images/6/64/BBa_K325219pheffect.xls Media:BBa_K325219pheffect.xls] | Raw data from experiment | 21/10/2010 |
Protocol
- The protocol can be found as Experiment 110.
Compatibility
[http://partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=cell Chassis:] Device has been shown to work in Top 10 (Invitrogen)
Plasmids: Device has been shown to work on <partinfo>pSB1C3</partinfo>
References
[http://www.ncbi.nlm.nih.gov/pubmed/18949818 [1]:] S.M. Marques and J.C.G. Esteves da Silva, (2009) Firefly Bioluminescence: A Mechanistic Approach of Luciferase Catalyzed Reactions,Life 61, 6-17.
[http://www.nature.com/nature/journal/v440/n7082/abs/nature04542.html [2]:] T. Nakatsu et al. (2006) Structural Basis for the spectral difference in luciferase bioluminescence, Nature 440(16), 372-376.
[http://www.ncbi.nlm.nih.gov/pubmed/11457857 [3]:] K. Gomi and N. Kajiyama, (2001) Oxyluciferin, a Luminescence Product of Firefly Luciferase, Is Enzymatically Regenerated into Luciferin, The Journal of Biological Chemistry, 276(39), 36508-36513.
 "
"