Team:HKU-Hong Kong/Project
From 2010.igem.org

Several ideas on the subject of this year's investigation were come up during various brainstorming sessions. Our team picked out the idea of engineering a mechanism that can act as a safety net to prevent genetically modified bacteria from performing undesired tasks in wrong environments.
Bacteria are genetically engineered to perform various functions.Prospective functions include biodegradation of crude oil and killing of cancer cells. Yet, undesired tasks might be performed by the baterium itself as well. The idea of a "bio-safety" net would serve the purpose.
For example, after fulfillment of intended functions or when bacteria have escaped from the intented work zone.
Contents |
Overall project
ways to achieve this
- Insertion of killing genes
- Regulated by specific promoters
- Promoters respond to changes in the environment
Hence bacteria can and can only function well and survive in a specific environment
Project Details
Killing Mechanism
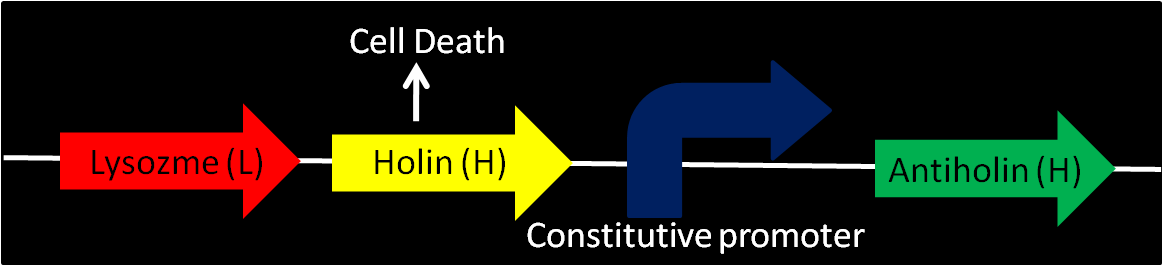
Enterobacteriophage T4 Lysis Device
- We found the biobrick form Berkeley's team from iGEM 2008 [Part:BBa_K112808, with no promotor] a possible way to attain cell destruction.
- Uses genes from enterobacteriaphage T4 (endolysin – lysozyme, holin and antiholin).
- T4 Holin (Length:676 bp):
- inner membrane-bound proteins, create pores in the membrane.
- T4 Antiholin (Length:311 bp):
- binds to and inactivates holin, producing an inactive holin-antiholin dimer.
- Lysozyme: T4 endolysin (Length:514 bp):
- enters the periplasm and degrades peptidoglycan, resulting in cell lysis.
- Various promoters can be installed into this device to control lysis.
- Antiholin has its own constitutive promoter to prevent formation of holin multimers from basal expression. When device is off, higher expression of antiholin is needed for better stability of the device.
- Others
- Lytic enzyme systems from bacteriophage
- Lambda
- phiX174
- MS2
- Qβ
- T2
- Hybrid designer lytic enzyme
- Lytic enzyme systems from bacteriophage
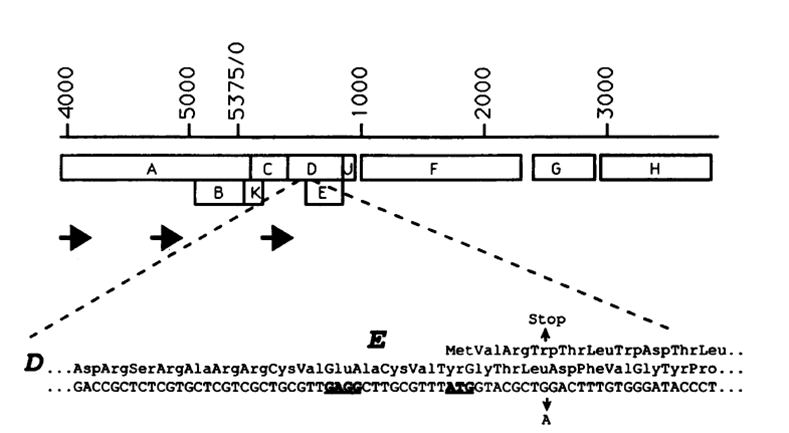
Gene E from Bacteriophage phiX174
The figure shows the location of gene E in the phiX174 genome (YOUNG, MICROBIOLOGIcAL REVIEWS, Sept. 1992,Vol. 56, No. 3)
- Gene E is the killing gene in the bacteriophage phiX174. Expression of gene E lead to lysis of the cell.
- Lysis works without elaborating a lytic enzyme, lysozyme
- Small, phase-dark spherical bulges or blebs were developed in host cells
- These blebs grow as big as the summed volumes of the residual rod-shaped portions of the host.
- Spherical bodies are freed, leaving empty and broken ghost
- They become less refractile and leaving visible membranous debris
- Some blebs burst and other phage release
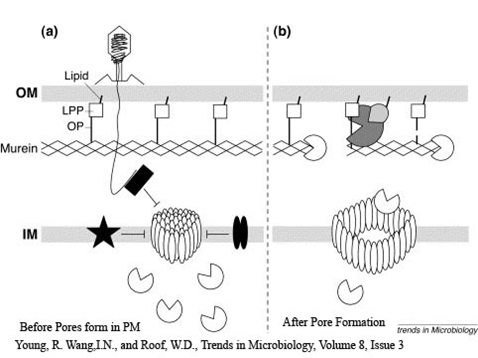
- Small lesions which are also called transmembrane tunnels started to appear
- Lesions cause destruction of inner and outer membrane
- Small holes at specific and distinct location in the cell formed
- Sudden collapse of membrane integrity
- The membrane potential and osmotic gradient across envelop collapsed
Changes in our plan
Difficulties
Oil is a mixture of chemicals composed mainly of hydrocarbons such as alkanes, carboxylic acids and various other aromatic compounds. As a direct result of this crude oil as a whole component cannot be relied upon to induce any promoter, since the promoters available to us may only be induced by certain chemical compounds (not a mixture) and because it is very difficult to target a specific component in crude oil. After assessing the feasibility of using a true oil degrading mechanism, we have concluded that it would be far too difficult to implement such a system into an E. Coli within the time period available to our project.
The changes
After identification of the difficulties, we decided to change our plan and instead of focusing on oil, we will use lactose to imitate ‘oil’. This lactose analogue can also be substituted with different subtracts with appropriate modification on the biobricks in initiating the mechanism. Lactose is used as the analogue owing to various reasons:
- Lactose operon is widely studied
- No reporter needed
- Lac-Z assay
- Optimal difficulty and achievable for 2 months’ time
So, now our plan has changed from making an oil digesting bacterium to a lactose digesting bacterium. After the digestion is done, the bacteria will kill themselves.
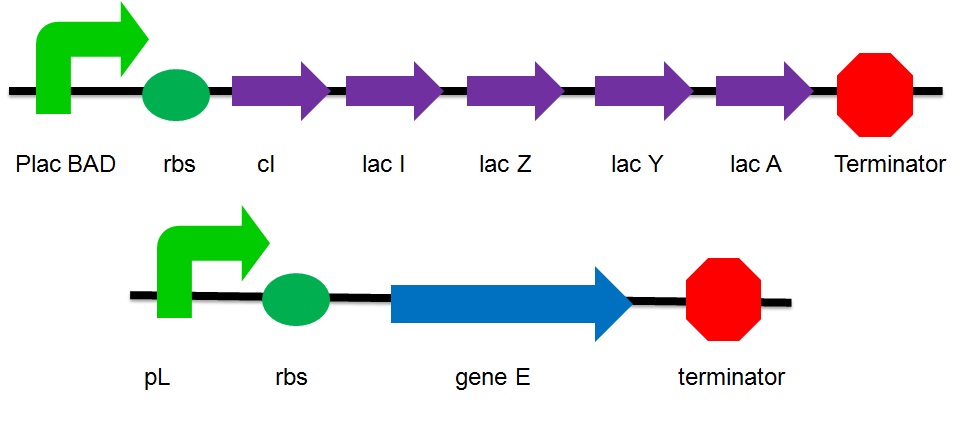
The plasmid design
- placBAD: It is the promoter of the lac operon inducible by lactose or arabinose. Without the substrate, the promoter is suppressed by the repressor protein. Under this condition, the gene is considered as turned off. No expression of genes that are upstream the suppressed promoter can be observed. No proteins that are encoded by these genes are produced.
- cI: It is the coding sequence of cI protein. The cI protein acts as a repressor protein and bind to the promoter of the suicide gene. The suicide mechanism is then turned off without expression of gene E and production of E protein.
- Lac I: Encode for the inhibitor of the lac operon
- Lac Z: Encode for Beta-galactosidase which breakdowns lactose to glucose & galactose and convert lactose to L-lactose which can activate the lac operon
- Lac A : Encode for lactose acetylase which transport lactose from medium to E.Coli
- Lac Y : Encode for lactose permease which regulates concentration lactose inside the cell
- pL : a promoter that can be inhibited by cI
Reasons for choosing lac operon as the “performed task”?
- Found in E.coli naturally
- Well-studied
- Easy to understand how mechanism of lac operon work
- Lactose as an analog of “crude oil molecule”
- Present in the chromosomes of E.coli, so recombination of a new promoter can be perfomed
Scenarios
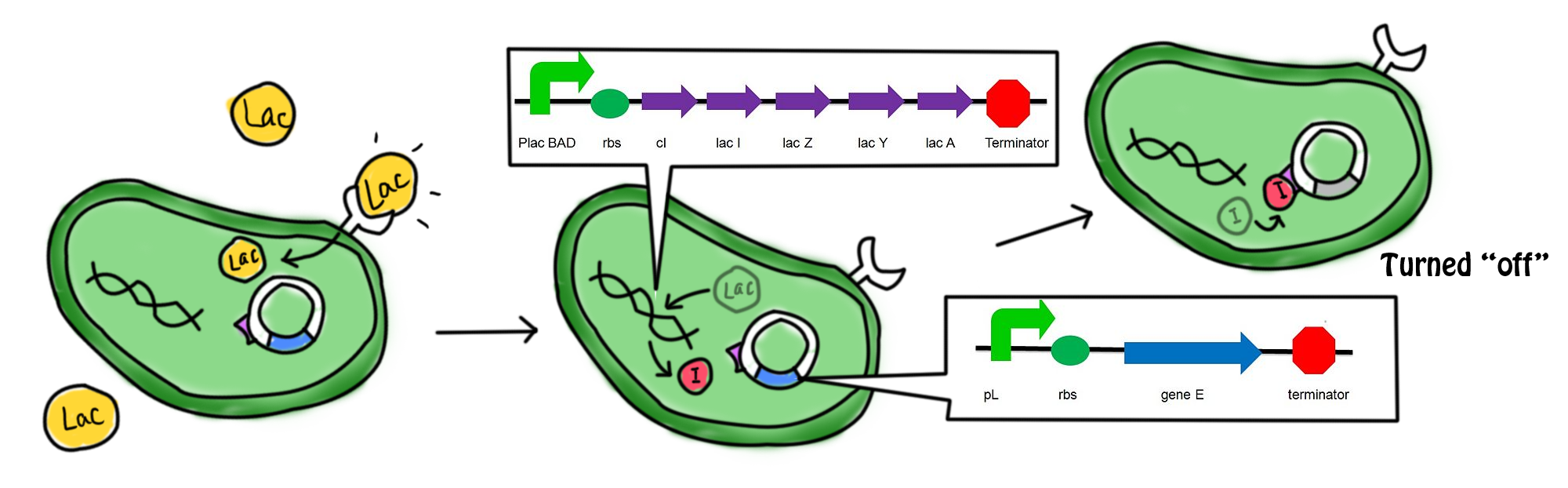
- In the presence of lactose, the lac operon and cI which were originally suppressed by repressor are now turned on. Transcription, translation and modifications are carried out to express the gene. cI protein and the β-galactosidase (hydrolytic enzyme that catalyzes the hydrolysis of lactose) are produced. The lactose starts to be digested. cI protein is the repressor of the promoter of suicide gene E, pL. The expression of suicide gene is therefore suppressed.
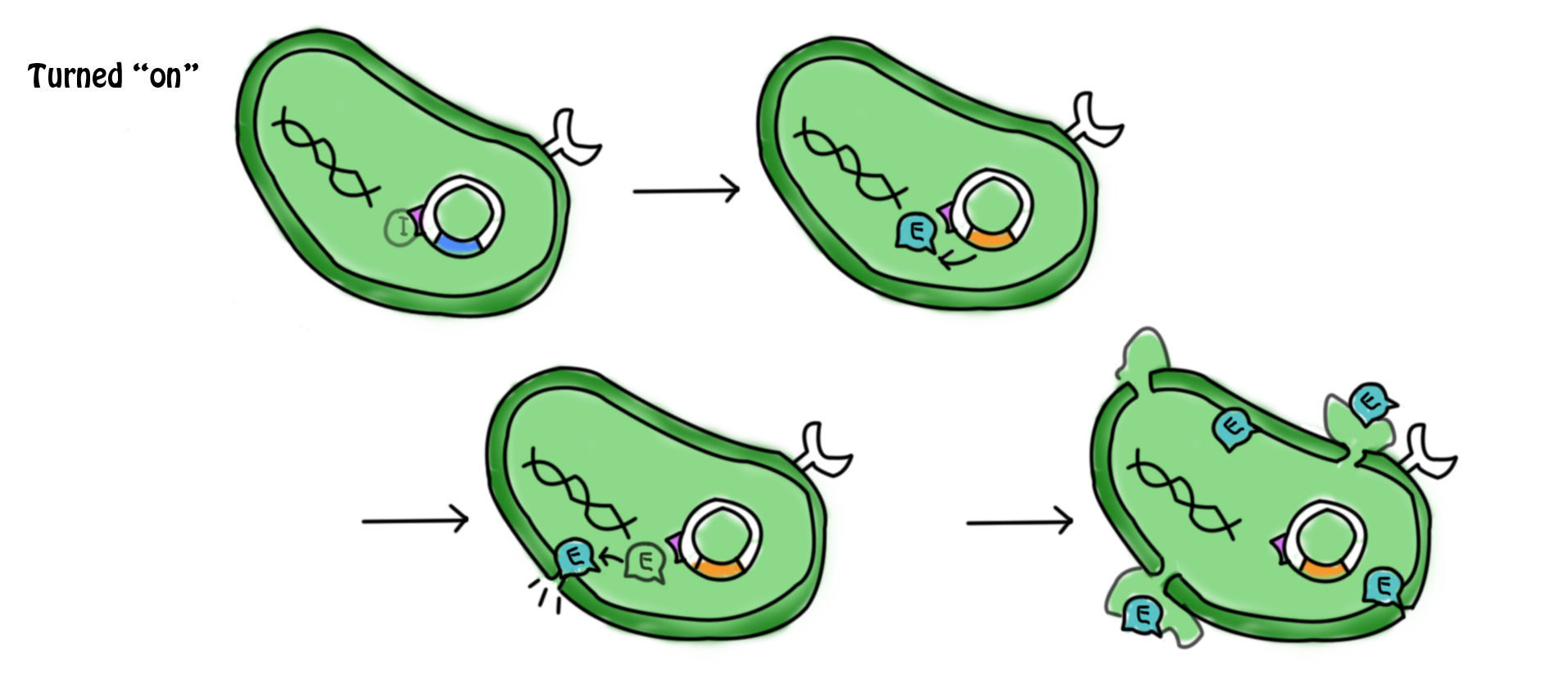
- The lactose in the cell culture is continuously digested. When all the lactose has been digested, the promoter of cI gene and lac operon are then suppressed. No β-galactozidase and cI protein are produced. In the absence of cI protein, the suicide gene is being turned on. The expression of gene E (production of E protein) leads to the formation of holes in the cell membrane which causes the cell death. The mechanism can be referred to the previous part.
- During the culture of the bacteria, arabinose is added to the LB agar which is the growing medium of the bacteria. The promoter of cI and lac operon can be activated by arabinose as well. Therefore, cI protein is produced continuously to suppress the expression of the suicide gene. This can prevent the bacterial cell death under un-desired conditions.
Possible flaws
- Leaky expression
- The suicide genes won’t work
- The time it takes for cell death
Results
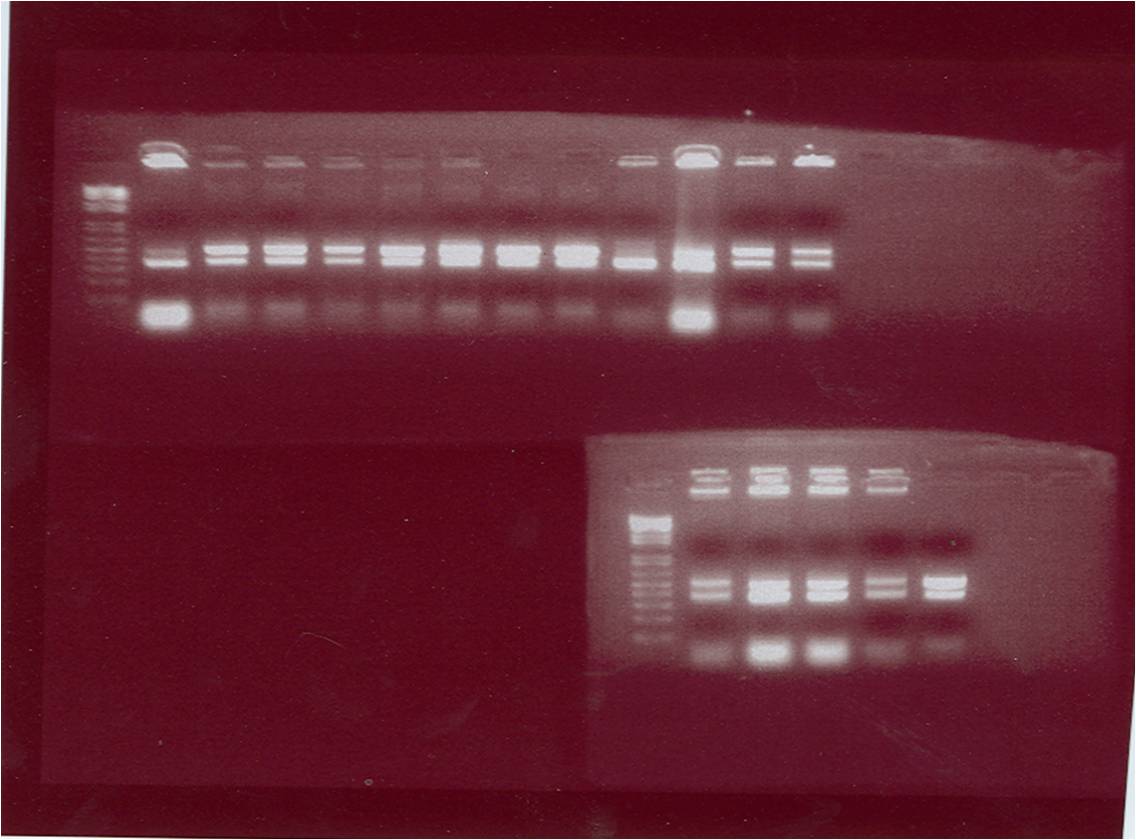
The figure above shows the gel photo of the suicide gene E The gel photo of the plasmid pET28a
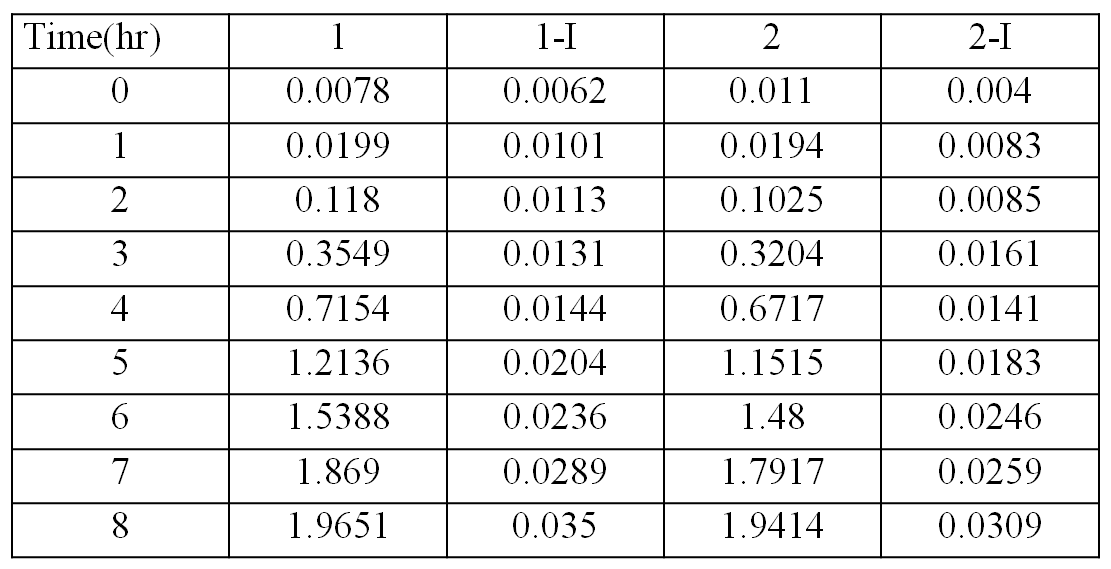
The table showing the optical densities of the bacterial culture with the bacteria contain transformed plasmids
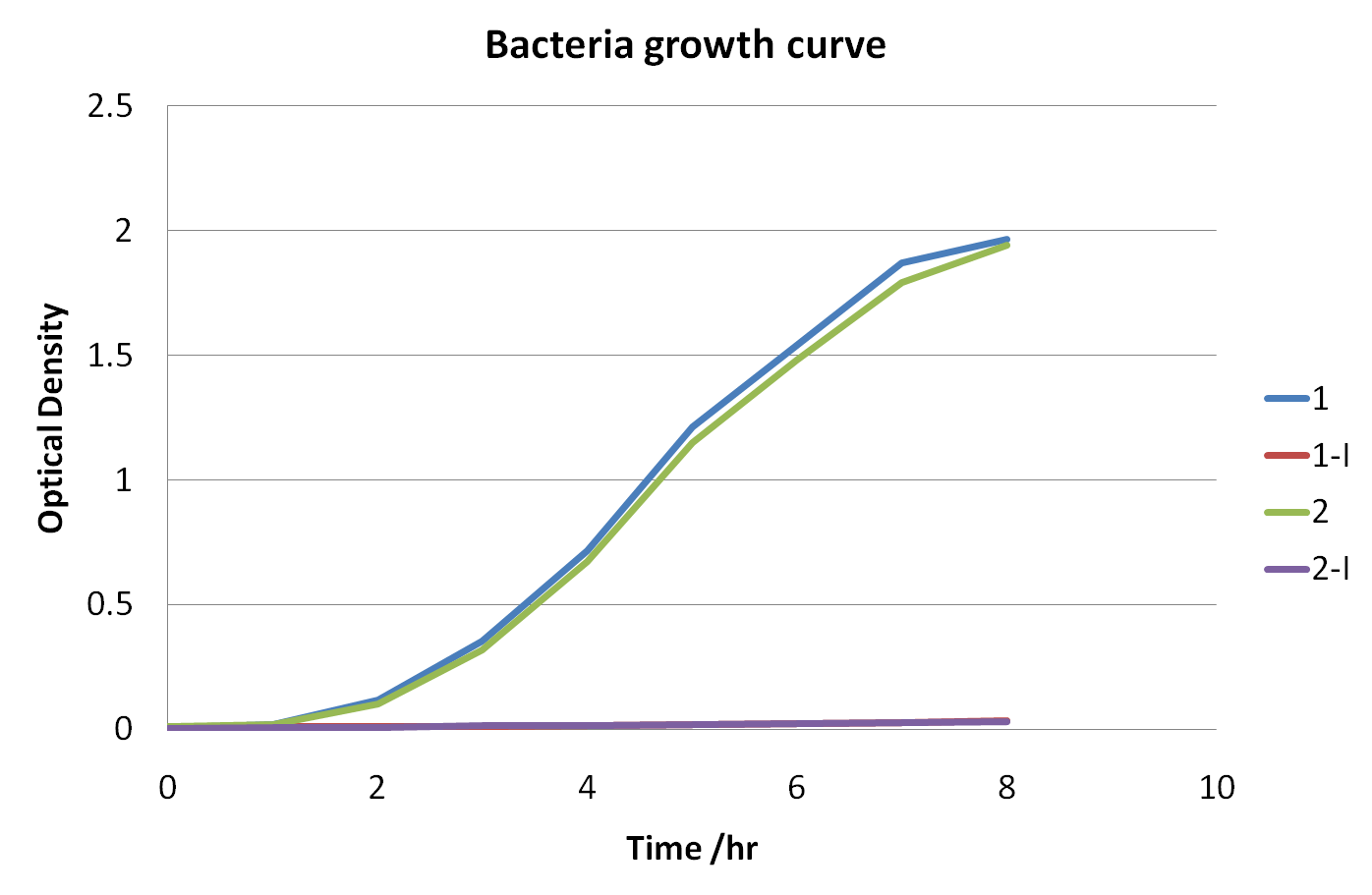
The graph showing the changes in optical density versus time
Possible further research
- The digestion part can be changed to any genes we want with different inducible promoters like heavy metal promoters.
- Ultimately we can technically make specific bacteria which digest different chemical and carry out suicide afterwards.
 "
"