Team:Michigan/Oil Sands October
From 2010.igem.org
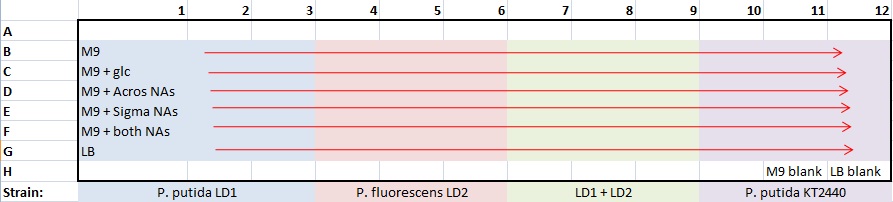
| Sunday | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | |
| Week 14 | - | - | - | - | - | 10/1/2010 | - |
| Week 15 | 10/3/2010 | 10/4/2010 | 10/5/2010 | - | 10/7/2010 | 10/8/2010 | - |
| Week 16 | - | - | - | - | - | - | 10/16/2010 |
10/1/2010
Alex
Moved broth cultures 37°C to 4°C -- 1:30pm (19-hr culture) Prepared biofilm assay plate for all pH9 tests - following Alex's biofilm assay protocol
- plate template:
- 100 μL/well: 99 μL medium + 1 μL culture
- stored plate in Lin Lab 4°C -- 2:20pm
Saved data for curves from ph7 plate Read pH7 plate ODs Performed biofilm assay on ph7 plate Started 24-hr kinetic reading for pH9 plate -- ~midnight
Discarded pH7 plate
Processed pH7 plate data
- uploaded results here
Uploaded pH7 kinetic data here
10/3/10
Alex
Ran CV biofilm assay on pH9 plate, following my protocol
- discarded plate
- uploaded results
- uploaded pH9 kinetic raw data
10/4/2010
Marcus and Ben
Performed ligation of Flu operon and pSB1AT3 according to the Ginko Bioworks Ligation protocol, with modifications.
- Calculated amount from digests to add to ligation for 1:1 and 3:1 insert:vector ratios
- Amount of DNA in digests:
- Flu PCR- 29.8 ng/uL x 17 uL= 506.6 ng / 50 uL = 10.132 ng/uL
- pSB1AT3- 240.5 ng/uL x 5 uL= 1202.5 ng / 50 uL = 24.05 ng/uL
- Used an online calculator to find moles:
- Flu PCR- 230.81 fmol/50 uL
- pSB1AT3- 537.68 fmol/50 uL
- This gives a molar ratio of 0.4293
- Amount of DNA in digests:
1:1 Ligation Mix
- 4 uL Flu PCR x 10.132 ng/uL= 40.528 ng
- 1.7 uL pSB1AT3 x 24.05 ng/uL= 40.885 ng
- 2 uL T4 Buffer
- 1 uL T4 Ligase
- 11.3 uL Ultra pure H2O
3:1 Ligation Mix
- 2 uL Flu PCR x 10.132 ng/uL= 20.264 ng
- 2.57 uL pSB1AT3 x 24.05 ng/uL= 61.809 ng
- 2 uL T4 Buffer
- 1 uL T4 Ligase
- 12.43 uL Ultra pure H2O
Note that the conversion factor for the 3:1 ligation mixture was applied in reverse, and thus it was actually a 1:3 insert:vector ratio.
Ligation program was the same as used in the Ligation protocol.
10/5/2010
Marcus
Performed transformation according to [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC145660/pdf/240536.pdf Pope & Kent (1996)].
Transformed Parts
- Flu/pSB1AT3 1:1 ligation
- Flu/pSB1AT3 1:3 ligation
- VP130
- J61100
- OmpA
- Cell only negative control
Procedure
- 2 uL of DNA was pipetted into PCR tube on ice and transferred to Lin lab
- Comp cells were thawed on ice and 50 uL was pipetted into each tube with a chilled pipette on ice
- Cells were pipetted up and down to mix throughly
- Cells were kept on ice and brought back to ERB
- Cells were plated directly onto pre-warmed plates with appropriate antibiotics
- Kevin put plates in incubator several hours before hand
It should be noted that two 200 uL aliquots of cells had to be thawed, and one was labeled "Thawed" and returned to the -80 C. Thawing should be avoided if possible.
Also, made two 5 mL LD1/LD2 co-cultures in M9+Sigma and Acros NAs, pH 7 and pH 9. Also made 2 mL pure cultures at pH 7. All culture tubes were stored in the 30 C. These cultures were made to assay growth, make new stock plates, and determine if colony morphology can be used to distinguish between species in a co-culture.
10/7/2010
Marcus
Ben moved transformation plates to 4 C yesterday, control plates were blank, and all plates except for J61100 appeared to have colonies. The Flu/pSB1AT3 ligation plates had very small colonies, so they were returned to the incubator with the J61100 plate. Also, it could be seen that many colonies on the 1:3 ligation plate appeared red, indicating that the vector had preferentially reformed. This could be because of the ratio, but dephosphorylation treatment should be used in the future for ligations.
OD600's were taken of the Pseudomonas cultures (46 hours)
- Putida pH 7- 0.157
- Fluorescens pH 7- 0.129
- Co-culture pH 7- 0.188
- Co-culture pH 9- 0.232
Surprisingly, they seem to grow better at pH 9, at least in co-culture. As expected, the co-culture did better than pure cultures. This lends some support towards Alex's biofilm assay results, however, it should still be repeated. Also, plated a serial dilution of the pH 9 co-culture, at 10-5, 10-6, and 10-7 dilutions to determine the conversion factor between OD600 and cell count. Made streak plates of all other cultures, pure cultures for stock, and pH 7 co-culture to determine if colonies could be distinguished. All cultures and plates were returned to the 30oC incubator, without shaking.
10/8/2010
Marcus
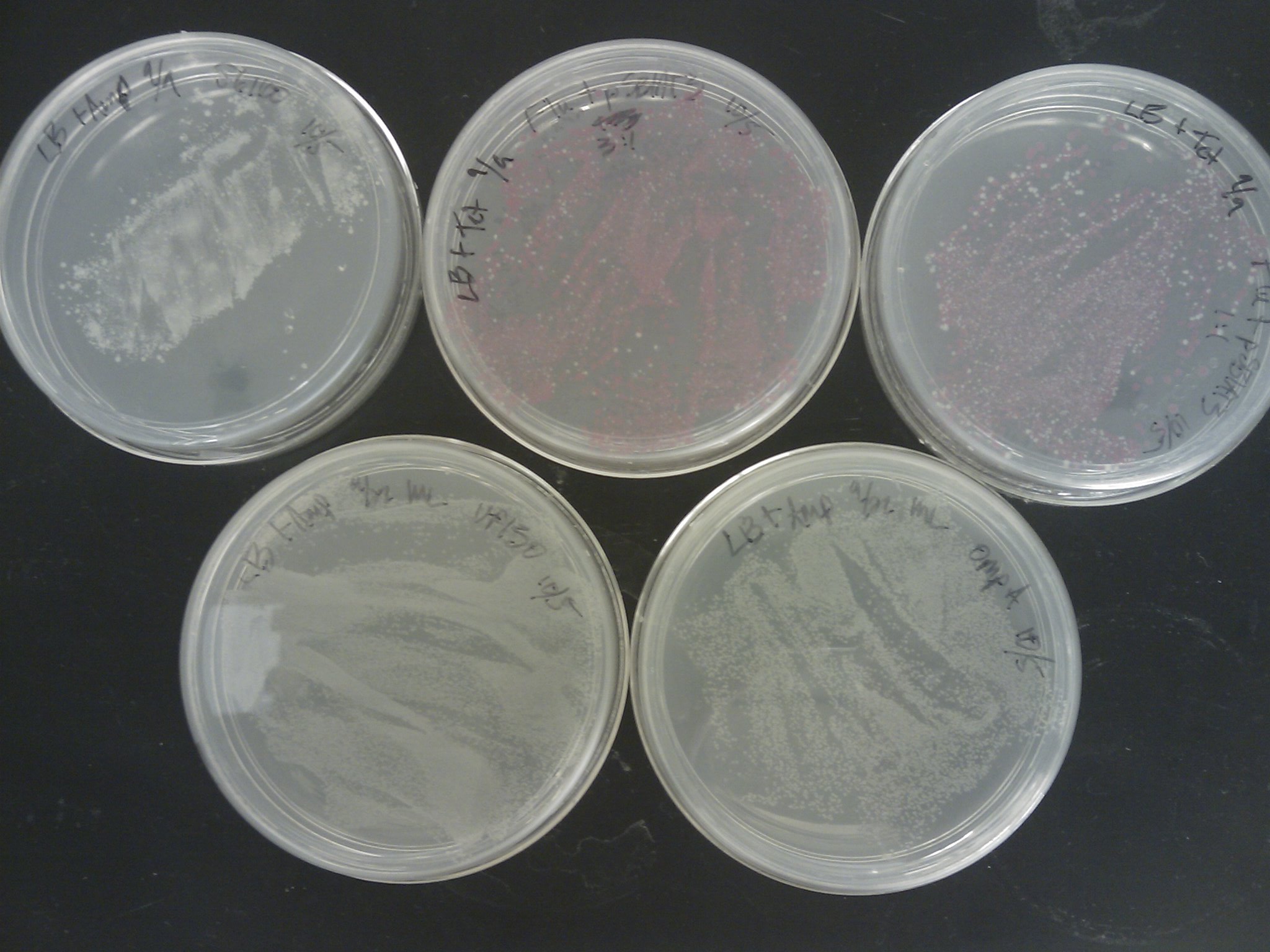
Kevin removed the transformation plates which were placed back in the incubator to the 4oC around 1 pm.
The [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC145660/pdf/240536.pdf Pope & Kent (1996)] appears to be very efficient, as the plates were full of colonies:
Next time suspending the cells in water before spreading, or even making dilutions should be tried.
PCR Screening: 8 colonies were picked from each transformation plate except for VP130, and suspended in 50 uL of water on a microplate.
- Plate Layout
- Row 1- Flu 1:1
- Row 2- Flu 1:3
- Row 3- OmpA
- Row 4- J61100
The first two rows (Flu 1:1 and 1:3) were PCR'd by adding 1 uL of each sample to a PCR tube with a drop of mineral oil in them. A master mix was made, and 50 uL was added to each tube (should have been 49 uL).
Master Mix (18x)
- 180 uL 5x Phusion Buffer
- 18 uL 10 mM dNTPs
- 45 uL VF2
- 45 uL VR
- 9 uL Phusion polymerase
- 585 uL Ultra pure H2O
PCR Program
- 1. 95 C for 10 min
- 2. 95 C for 30 sec
- 3. 62 C for 30 sec
- 4. 72 C for 2 min
- 5. Return to step two 34 times
- 6. 72 C for 10 min
- 7. 4 C store
This PCR protocol was constructed from the following sources:
- [http://openwetware.org/wiki/Engineering_BioBrick_vectors_from_BioBrick_parts/Colony_PCR_protocol OpenWetWare BioBrick Primers Colony PCR]
- [http://openwetware.org/wiki/Endy:Colony_PCR OpenWetWare Endy Colony PCR]
- [http://www.finnzymes.com/pdf/f530sl_phusion_datasheet_2_2_low.pdf Finnzymes Phusion Manual]
Also- loosened the lids on Pseudomonas liquid cultures to provide air exchange, and returned yesterday's Pseduomonas plates to 30C incubator, without shaking.
10/16/2010
Nick
10-16-10 Psuedomonas Competent Cell Preparation
Protocol - Competent Cell Preparation for Quick Transformation of E. coli
Ben Parker started the overnight cultures and assisted in OD measurement and initial centrifugation steps.
A colony of P. putida and P. fluorescence was picked from LB agar plates (10/6/10) and grown up in an overnight culture in 2ml Amp at 30 C.
The overnight culture was added to 100 ml of LB media and grown at 30C for 4 hours.
Protocol was carried out approximately 20 minutes after the following OD600 was recorded:
Blank - water
P. putida – 0.587
P. Fluorescence – 0.610
One centrifuge tube containing p. putida culture failed during centrifugation at 5000 rpm. The protocol was altered by reducing the speed to 2500 rpm after this point to reduce the chance of failure. The cells are stored in the 4C fridge in room 1239.
 "
"