|
PROTOCOLS
Media & Antibiotics
LB
- Add:
- 10 g/L NaCl
- 10 g/L Bacto-Tryptone
- 5 g/L Bacto-Yeast Extract
- ddH2O
to a sterile pyrex bottle
- autoclave
- (add antibiotic when it reaches ~45°C)
- store at +4°C
LB Agar
- Add:
- 10 g/L NaCl
- 10 g/L Bacto-Tryptone
- 5 g/L Bacto-Yeast Extract
- 15 g/L Bacto-Agar
- ddH2O
to a sterile 1L flask
- autoclave
- (add antibiotic when the agar becomes visible in the liquid, at about 45°C; shake gently to avoid bubbles)
- pour into Petri plates and close them immediately
- let them polymerize for ~2-3h
- invert plates and wrap them with aluminium foil and store at +4°C
SOB
- Add:
- 5 g/L Bacto-Yeast Extract
- 20 g/L Bacto-Tryptone
- 10mM NaCl
- 2.5mM KCl
- 10mM MgSO4
- 10mM MgCl2
to a sterile pyrex bottle
- (optional: check that pH is ~6.8, otherwise adjust with NaOH)
- autoclave
- (add antibiotic when it reaches ~45°C)
- store at +4°C
SOC
- Prepare SOB and add 20 ml of 1M glucose (prepare it dissolving 3.6 g of glucose in 20 ml of ddH2O).
M9 supplemented with glycerol (M9gly)
For 1L of medium, add:
- 734 ml of autoclaved (and cooled to Tamb, N.B. consider evaporation during autoclaving) ddH2O with a magnetic stirrer inside the bottle
- 5 ml of autoclaved 80% glycerol as carbon source
- 100 ul of autoclaved or filtered (0.2um) CaCl2 1 M
- 20 ml of 10% autoclaved casamino acids (dissolve 50 g in 500 ml = 10% stock)
- 34 ml of filtered (0.2um) thiamine hydrochloride MW=337.27g/mol (340 mg in 34 ml) (keep in mind it is photosensitive)
- 2 ml of autoclaved MgSO4 1 M
- 200 ml of autoclaved M9 salts 5x (dissolve 56.4 g in 1 liter ddH2O = 5x stock)
- shake the ddH2O with the magnetic stirrer and start adding the other solutions in sterility (each solution must be completely dissolved!) in the order listed above.
- add antibiotic if needed
- store at +4°C, protected from light
NOTE:
- M9 salts 5x, 10% casamino acids, MgSO4 1 M and CaCl2 1 M can be stored at +4°C.
- glycerol 80% can be stored at room temperature.
- thiamine hydrochloride (LIGHT SENSITIVE) is one-shot and must be prepared each time (keep in mind you loose some volume during filtration)
Antibiotics
Stocks at -20°C freezer:
- Ampicillin 100 mg/ml (in water)
- Kanamycin 50 mg/ml (in water)
- Chloramphenicol 34 mg/ml (in 100% ethanol)
These stocks are 1000x for high copy number plasmids.
For low copy number plasmids, you should use these final concentrations in media:
- Ampicillin 50 ug/ml
- Kanamycin 20 ug/ml
- Chloramphenicol 12.5 ug/ml
E. coli transformation
Transforming home-made competent cells
- heat ligation at 65°C to inactivate T4 ligase
- thaw in ice a vial of TOP10 competent cells stored at -80°C
- incubate a selective LB agar plate at 37°C
- pipet 800ul of LB (without antibiotic) in a 15ml falcon tube and incubate it at 37°C
- heat the water bath at 42°C
- add 1 ul (~3ng of DNA vector) of ligation to 100ul of thawed TOP10
- add parafilm and incubate in ice for 30 min
- heat shock at 42°C for 1 min
- incubate in ice for 2 min
- transfer transformed bacteria to 800ul of pre-warmed LB
- incubate at 37°C, 220 rpm for 1 h
- centrifuge at 2000 rpm, 25°C for 10 min
- take 750ul of supernatant and resuspend the pellet in the remaining LB (~150ul)
- plate the entire culture and incubate the plate at 37°C overnight
Variants:
- if you transform a miniprep, add less than 3 ng in order to have single colonies
- if you use another home-made competent strain, the protocol is the same but you should consider the transformation efficiency to add a proper amount of DNA
- if you use commercial Invitrogen TOP10 the protocol changes and it is reported below.
Transforming commercial competent cells
(according to manufacturer’s protocol)
- heat ligation at 65°C to inactivate T4 ligase
- thaw in ice a vial of TOP10 competent cells stored at -80°C (one vial contains 50ul of cells)
- incubate a selective LB agar plate at 37°C
- heat the water bath at 42°C
- dilute the ligation 1:50 (or 1:100) in ddH2O, in order to have less than 100pg/ul
- add 1 ul of ligation (or less than 100pg of miniprepped DNA) to 25 or 50ul of thawed TOP10
- add parafilm and incubate in ice for 10 min
- heat shock at 42°C for 1 min
- incubate in ice for 2 min
- add 250ul of SOC medium
- incubate at 37°C, 220 rpm for 1 h
- plate 150ul of the culture and incubate the plate at 37°C overnight
- the remaining 150ul can be stored at +4°C
E. coli competent cells preparation
H. Inoue et al. (1990), High efficiency transformation of Escherichia coli with plasmids, Gene 96 23-28.
- DAY1
- inoculum 5-8 ul from -80°C stock in 5 ml of LB (37°C, 220 rpm ON);
- DAY2
- dilution 1:1000 in SOB (flask, 18-25°C, 220 rpm ON);
- DAY3
- pre-chill centrifuge at 4°C;
- prepare TB (prepare 50 ml every 125 ml of SOB):
- 15mM CaCl2
- 250mM KCl
- 10mM (3 g/L) Pipes
- adjust pH at 6.7 with KOH
- 55mM (8.9 g/L) MnCl2
- filter (0.2 um) the solution and chill in 50 ml
- put the flask in ice when the culture reaches OD600=~0.05 (1mm pathlength – NanoDrop);
- aliquot in pre-chilled 50 ml falcon tubes;
- centrifuge at 2500g (4400rpm), 4°C, 10 min;
- ICE: discard, resuspend in 40 ml of TB each 125 ml SOB, centrifuge as before;
- ICE: discard, resuspend in 10 ml of TB each 125 ml SOB, add 700ul DMSO;
- ICE: aliquot 100ul in pre-chilled 0.5ml tubes;
- put in -80°C freezer;
ALWAYS TEST THE EFFICIENCY IN [CFU/ug] UNITS: transform 100ul of competent cells with 4ng of DNA and 100ul of competent cells without DNA (add 1ul of ddH2O), then plate on proper LB agar plates.
This protocol has shown to work with:
- DH5alpha (10^8 with 100ul of cells);
- TOP10 (5*10^7 with 100ul of cells);
- BW20767 (10^3 with 100ul of cells);
- DB3.1 (5*10^4 with 100ul of cells);
J. Sambrook, E.F. Fritsch, T. Maniatis (1989)
- DAY1
- Inoculum 5-8 ul from -80°C stock in 5 ml of LB (37°C, 220 rpm ON).
- DAY2
- Dilution 1:500 in LB (flask, 30-37°C, 220 rpm), monitor OD600 until it reaches 0.04 (1mm pathlength – NanoDrop, it should take from 3 to 5 hours);
- prepare the proper amount of 50 ml tubes in ice and pre-chill the centrifuge;
- when the culture reaches the right OD600, aliquot the culture in the pre-chilled tubes;
- centrifuge (4000rpm, 4°C, 10min) and discard the supernatant;
- for each 50 ml of culture, add 30 ml of MgCl2-CaCl2 solution (Buffer1) and resuspend the pellet;
- centrifuge (4000rpm, 4°C, 10min) and discard the supernatant;
- for each 50 ml of the original culture, add 2 ml of CaCl2 solution (Buffer2) and resuspend the pellet;
- aliquot in 0.5 ml tubes and store at -80°C.
ALWAYS TEST THE EFFICIENCY IN [CFU/ug] UNITS
- Buffers preparation
- Buffer1: 80mM MgCl2, 20mM CaCl2 (e.g.: mix 8 ml MgCl2 1M, 2 ml CaCl2 1M and 90 ml ddH2O):
- put ddH2O into a flask or a bottle and autoclave it;
- add MgCl2 previously filter-sterilized (0,2 um) and CaCl2 previously autoclaved or filter-sterilized (0,2 um).
- Buffer2: 0.1 M CaCl2 and 15% of glycerol (e.g.: mix 100 mL of 1M CaCl2, 150 mL of 100% Glycerol and 750 mL of ddH2O):
- put ddH2O and glycerol into a flask and autoclave it;
- add CaCl2 previously autoclaved or filter-sterilized (0,2 um).
Keep Buffers cold.
E. coli strains (all in -80°C freezer)
TOP10
F- mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 nupG recA1 araD139 Δ(ara-leu)7697 galE15 galK16 rpsL(StrR) endA1 λ-
- competent cells already prepared (5*10^7 CFU/ug with100ul of cells)
- competent cells from Invitrogen available (10^9 CFU/ug with 50ul of cells)
- commonly used for cloning and expression in our lab
- they are equal to DH10B strain, whose genome is available from NCBI
NOTE: they have
- lacI wt
- cI of phi80 prophage (different from cI of lambda phage)
- Streptomycin resistance
DH5alpha
F- endA1 glnV44 thi-1 recA1 relA1 gyrA96 deoR nupG Φ80dlacZΔM15 Δ(lacZYA-argF)U169, hsdR17(rK- mK+), λ–
- competent cells already prepared (10^8 CFU/ug with100ul of cells)
- commonly used for cloning
BW20767
F-, RP4-2(Km::Tn7,Tc::Mu-1), leu-163::IS10, ΔuidA3::pir+, recA1, endA1, thi-1, hsdR17, creC510
- competent cells already prepared (10^3 CFU/ug with100ul of cells)
- not used for cloning
NOTE: they have
- a fully working lac operon (already tested on IPTG/X-Gal plates)
- Kan and Tet resistance (not tested)
XL1-Blue
endA1 gyrA96(nalR) thi-1 recA1 relA1 lac glnV44 F'[ ::Tn10 proAB+ lacIq Δ(lacZ)M15] hsdR17(rK- mK+)
- competent cells never prepared
- a small stock of competent cells is available
- used for cloning
NOTE: they have lacIQ
DB3.1
F- gyrA462 endA1 glnV44 Δ(sr1-recA) mcrB mrr hsdS20(rB-, mB-) ara14 galK2 lacY1 proA2 rpsL20(Smr) xyl5 Δleu mtl1
- competent cells already prepared (5*10^4 CFU/ug with 100ul of cells)
- used for in vivo amplification of ccdB plasmids
NOTE: they have a working lacZ, but a deleted lacY, they become slightly blue on IPTG/X-Gal plates
STBL3
F- glnV44 recA13 mcrB mrr hsdS20(rB-, mB-) ara-14 galK2 lacY1 proA2 rpsL20 xyl-5 leu mtl-1
- competent cells never prepared
- used for in vivo amplification of DNA with direct repeats
NOTE: they cannot be used for blue/white screening
CW2553 + pJat8
Genotype: Khlebnikov A et al. (2000), Regulatable Arabinose-Inducible Gene Expression System with Consistent Control in All Cells of a Culture, Journal of Bacteriology, Vol. 182, No. 24, p.7029-7034.
- pJat8 is Gentamycine resistant
NOTE:
- the stock of this strain has been grown without Gen
- this strain is used for araBAD inducible system
MG1655 (seq)
F-, λ-, rph-1
- CGSC#7740
- Fully sequenced genome (GenBank: NC_000913)
MC1061
F-, Δ(araA-leu)7697, [araD139]B/r, Δ(codB-lacI)3, galK16, galE15(GalS), λ-, e14-, mcrA0, relA1, rpsL150(strR), spoT1, mcrB1, hsdR2
BW25141
F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), Δ(phoB-phoR)580, λ-, galU95, ΔuidA3::pir+, recA1, endA9(del-ins)::FRT, rph-1, Δ(rhaD-rhaB)568, hsdR514
- CGSC#7635
- Carry the wild type pir gene
BW25142
F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), Δ(phoB-phoR)580, λ-, galU95, ΔuidA4::pir-116, recA1, endA9(del-ins)::FRT, rph-1, Δ(rhaD-rhaB)568, hsdR514
- CGSC#6649
- Carry the pir-116 gene
BW23473
F-, Δ(argF-lac)169, ΔuidA3::pir+, recA1, rpoS396(Am)?, endA9(del-ins)::FRT?, rph-1, hsdR514, rob-1, creC510
- CGSC#7837
- Carry the wild type pir gene
BW23474
F-, Δ(argF-lac)169, ΔuidA4::pir-116, recA1, rpoS396(Am)?, endA9(del-ins)::FRT, rph-1, hsdR514, rob-1, creC510
- CGSC#7838
- Carry the pir-116 gene
Long term bacterial glycerol stocks
- Mix 750 ul of a culture (preferably in log-phase) with 250 ul of 80% glycerol, in a 1.5ml vial
- label the vial with name, date and antibiotic resistance
- leave at -20°C for one day
- move to -80°C the day after
Plasmid digestion for BioBrick Standard Assembly
- To open vectors
-
- a volume containing 1 ug of purified plasmid
- 2.5 ul of 10X buffer H
- 1 ul of first enzyme
- 1 ul of second enzyme
- 25 ul final volume
- incubate at 37°C for 3 hours
- To excide fragments
-
- A volume containing 1-1.8 ug of purified plasmid
- 2.5 ul of buffer H
- 1 ul of first enzyme
- 1 ul of second enzyme
- 25 ul final volume
- incubate at 37°C for 3 hours
NOTE: if you are performing a digestion for screening, 1 hour of incubation is sufficient.
Ethanol precipitation with sodium acetate
- Add 1/10 DNA solution volume of sodium acetate 3 M, pH 5.2
- Add 2.5 DNA solution volume of absolute ethanol
- Freeze at -80°C for 30 min
- Centrifuge at 13000 rpm, 4°C for 20 min
- Decant supernatant
- Add 250 µl of 70% ethanol
- Centrifuge at 13000 rpm, 4°C for 20 min
- Remove all supernatant with a pipette
- Air dry pellet until ethanol is totally removed
- Elute with 5-10 µl of ddH2O
Ligation
After the purification of two digested DNA fragments:
- add a volume containing 20-50 ng of vector
- add a volume containing:
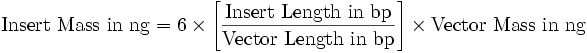
(“6” can be lowered to “2”)
- heat DNA mix at 65°C for 5 min for DNA denaturation
- add 1 ul of T4 Ligase buffer (check if ATP is completely dissolved)
- add 1 ul of T4 Ligase
- 10-20 µl final volume
- incubate at 16°C overnight
- inactivate the T4 Ligase heating at 65°C for 10 min
- then, ligation can be conserved at 4°C or can be transformed
NOTE:
When the purified DNA of the insert also contains its native vector, you can perform the ligation anyway, but its antibiotic resistance must be different from the acceptor vector’s resistance in order to select correct transformants on agar plates.
When doing this, you should modify the ligation protocol:
- you should use “2” or “3” instead of “6” to compute the insert mass;
- when you add the volume containing the insert mass, you must consider that the DNA quantification with NanoDrop refers to insert+NATIVE VECTOR. So, you must add:
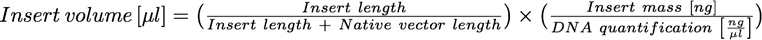
DNA resuspension from iGEM plates
- Find the right position of the DNA of interest in iGEM plates
- Resuspend with 15 ul ddH2O and transfer it in 0,5 ml sterile Eppendorf tubes
Now you can store them at -20°C or transform in your favorite strain.
PCR
- For every DNA sample you want to amplify, put:
- 2 µl buffer
- 0.6 µl MgCl2
- 0.4 µl dNTPs
- 1 µl DNA (or ddH2O for blank sample). If you are performing a colony PCR, pick up the desired colony from a plate with a tip and dip it in the solution.
- 0.25 µl Taq Polymerase
- 0.5 ul VF2 primer (10 uM)
- 0.5 ul VR primer (10 uM)
- A proper amount of ddH2O to have 20 µl of total reaction volume
- into an eppendorf tube.
- Put the Eppendorf tube in the thermal cycler and set this program:
- 95°C 10 min
- CYCLE:
- 95°C 30 sec
- 60°C 1 min
- 72°C 1-3 min
- for 35 cycles
- 72°C 7 min
- 16°C forever.
- Now you can add a loading buffer to the solution and perform electrophoresis to check the amplified sequence length.
Electrophoresis
- Prepare agarose gel in 1x TBE buffer
- Add ethidium bromide (using gloves and face mask for your safety):
- 1,5 µl in the small size agarose gel (70 ml)
- 3 µl in the middle size agarose gel (150 ml)
- 5 µl in the big size agarose gel (250 ml)
- Cast the gel, insert the well-forming comb and let it polymerize
- Add the loading buffer (10x Blue Juice, Invitrogen) to each sample
- Load the samples and 8 µl of marker (when not specified: 1 kb Plus DNA Ladder, Fermentas)
- Set to 70-100 volts and electrophorese for the required amount of time
- Use UV-light to look at the bands (using gloves and protective glasses)
- Take a picture of the gel, if needed (not when bands have to be cut!!!)
|
|
1 kb Plus DNA Ladder preparation (Fermentas)
Mix gently:
- 1ul of DNA ladder (1 kb)
- 1ul of 6X DNA Loading Dye
- 4ul of Deionizied water
A final volume of 6ul to load
Glycerol stocks
- Mix 750 ul from the 5 ml sample of the incubated bacteria with 250 ul of 80% glycerol
- Leave at -20°C for one day
- Move to -80°C the day after
X-Gal staining protocol for beta galactosidase (blue/white screening)
- The principle is that X-Gal (5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside) turns blue when reacts with beta-galactosidase.
- Mix 20 ul X-Gal 40 mg/ml and 60 ul LB and spread on required LB agar plates (X-Gal and DMF are toxic, use face-mask for your safety!!! X-Gal is light-sensitive, remember to keep it in the dark, when possible).
- If you have to induce beta-galactosidase production (for example in lac operon) add 20 ul of IPTG 200 mM to the mix.
- Let plates dry at 37°C and than plate bacteria.
- Incubate at 37°C.
- Result: blue colonies express LacZ, while white colonies don't.
Sudan Black staining protocol
- Take 70 ul of colture and spread it onto a slide.
- Allow the smear on the slide to dry.
- Heat fix the slide by passing it through a Bunsen burner flame.
- Place a few drops of Sudan Black solution (0,3% in 70% ethanol) on the fixed preparation.
- Leave the solution work for 10 minutes till the ethanol in the stain is evaporated.
- Immerse the slide in the xylene for 10 seconds to allow the decolorization.
- Add Safranine solution (0,5% in wather) to the slide and leave it for 10 seconds to allow the counter-staining.
- Wash the slide with running water.
- When the slide is completely dry, add a drop of immersion oil directly to the slide.
- Examine the slide with optical microscope with 100x oil immersion objective.
Yeast cultures
Liquid YPD medium (0.5 L)
- 5 g yeast extract
- 10 g peptone
- 450 ml ddH2O
- Autoclave
- Add 50 ml of 20% glucose to reach the final concentration of 2%
- Add 2 ml of G418 geneticin (50 mg/ml stock) at the final concentration of 200 ug/ml
YPD agar (0.5 L)
- 5 g yeast extract
- 10 g peptone
- 10 g agar
- 450 ml ddH2O
- Autoclave
- Add 50 ml of 20% glucose to reach the final concentration of 2%
- Add 2 ml of G418 geneticin (50 mg/ml stock) at the final concentration of 200 ug/ml
G418 stock solution (50 mg/ml)
- Add 20 ml of ddH2O to 1 g of G418 powder (Sigma)
- Filter-sterilize (0.2 um) and aliquot in 1 ml stocks
- Store at +4°C as recommended by Sigma
LiAc 1M
- Dissolve 1 g of LiAc dihydrate (Sigma) in ddH2O to a final volume of 10 ml
- Filter-sterilize (0.2 um) and store at +4°C
PEG 3350 50%
- Dissolve 20 g of PEG 3350 (Sigma) in ddH2O on a magnetic stirrer to a final volume of 40 ml
- Autoclave
- Store at room temperature
Long term glycerol stocks
- Mix 810 ul of an overnight yeast culture with 190 ul of sterile 80% glycerol
- Vortex and store at -80°C
Yeast transformation
- Follow this protocol: http://openwetware.org/wiki/High_Efficiency_Transformation until “Pipette
1.0 ml of sterile water into each tube; stir the pellet by with a micropipette tip and vortex” step.
- Centrifuge at 3000g 5 min, remove the supernatant and inoculate the pellet in 1 ml of pre-warmed
YPD in a 15 ml falcon tube.
- Incubate the cultures at 30°C, 200rpm for 2h.
- Centrifuge at 3000g 5 min, remove the supernatant and resuspend the pellet in the remaining
YPD.
- Plate the whole cells on a G418 plate, pre-incubated at room temperature.
- Incubate the plated cells at 28-30°C until colonies appear.
|